India Globalization Capital, Inc. (NYSE: IGC) Breaking News -
January 31, 2018
Inherent Nature of Blockchain
Technology Opens Door for New Industry Applications
New York, NY – January 31, 2018 –
NetworkNewsWire.com News Coverage:
Blockchain and cannabis stocks enjoyed an impressive market run in 2017 and
industry analysts expect the trend to continue in 2018. As these industries
continue to grow, innovators are finding ways to integrate this growth and
address areas of need in different verticals. Case in point,
 India
Globalization Capital, Inc. (NYSE: IGC), the developer of a
patent-pending cannabis-based combination therapy for Alzheimer’s disease,
recognizes the need for accurate labeling of cannabidiol products online. In
response, the company is developing blockchain technology-based solutions for
the cannabis industry. With this endeavor, the company is participating in the
rampant growth and application of blockchain benefits, alongside Eastman Kodak
Company (NYSE: KODK), BTL Group Ltd (OTC: BTLLF), Riot Blockchain, Inc. (NASDAQ:
RIOT) and China Information Technology, Inc. (NASDAQ: CNIT). India
Globalization Capital, Inc. (NYSE: IGC), the developer of a
patent-pending cannabis-based combination therapy for Alzheimer’s disease,
recognizes the need for accurate labeling of cannabidiol products online. In
response, the company is developing blockchain technology-based solutions for
the cannabis industry. With this endeavor, the company is participating in the
rampant growth and application of blockchain benefits, alongside Eastman Kodak
Company (NYSE: KODK), BTL Group Ltd (OTC: BTLLF), Riot Blockchain, Inc. (NASDAQ:
RIOT) and China Information Technology, Inc. (NASDAQ: CNIT).
In December 2017, heavy investor trading in cannabis and blockchain stocks
helped to push the profits of companies in these sectors higher. TD Ameritrade
Holding Corp. reported January 23 that daily average trades for the last quarter
of 2017 were up 49 percent from the previous year, with trades in the blockchain
and cannabis sectors accounting for between 6 and 9 percent of daily activity
(http://nnw.fm/7TfXC).
This heightened interest in cryptocurrencies ripples far beyond TD Ameritrade’s
portfolio, however, and has led to widespread application of the underlying
blockchain technology to a diverse range of industries, including cannabis.
IGC Adds Blockchain Technology to its Cannabis Platform
A recent study published in JAMA (http://nnw.fm/7hJdD) showed that almost 70
percent of cannabidiol (“CBD”) products sold online are incorrectly labeled.
Recognizing this issue as an opportunity,
India Globalization Capital, Inc. (NYSE: IGC) in late December 2017
announced its plans to develop ways of using blockchain technology for Product
Identification Assurance (PIA) of its cannabidiol (CBD)-based therapies. The
following day, IGC’s stock was up by over 200 percent and selling at $1.26 a
share.
Shortly after, SeeThruEquity issued an update on IGC and raised its price target
on the company’s stock to $2 a share, noting the company’s new and existing
initiatives.
There are currently four proprietary cannabis-based products in IGC’s pipeline,
including the company’s lead product, Hyalolex, for the treatment of Alzheimer’s
disease. The formulation of this flagship candidate includes the psychoactive
cannabinoid tetrahydrocannabinol (THC), which works in combination with other
active ingredients to slow the buildup of plaque in the brain.

IGC has defined a two-part commercialization strategy for Hyalolex (http://nnw.fm/5Q3bx):
follow the traditional FDA pathway through registered preclinical and clinical
trials; and license its formulation technology as a Complementary and
Alternative Medicine (CAM) to cannabis dispensaries in the United States.
Initial distribution is geared toward licensed medical cannabis dispensaries in
Maryland, Washington, D.C., and California.
The latter process will include state-by-state sourcing, formula assembly, and
packaging and distribution, utilizing best practices to ensure quality control
while meeting the legal guidelines of each state in which Hyalolex is sold.
Importantly, IGC plans to develop its blockchain platform to bring Hyalolex to
market, and from there leverage the advantages of the technology to address
inadequate product labeling, transactional difficulties, product origin and
other broad industry challenges.
Moving forward with its plan to develop blockchain-based methods for PIA and
other industry challenges, IGC will utilize the expertise of its technology and
health care experts to achieve an end-goal of producing an independent,
licensable product.
“We understand the unique challenges facing the cannabis industry and believe
that our team has the expertise to be the first to create meaningful solutions
to address these issues using distributed ledgers inherent in blockchain
technology,” IGC CEO Ram Mukunda stated in the press release (http://nnw.fm/J8Z8p).
“As we work to develop blockchain in the rollout of Hyalolex, our goal would be
to establish a universal cannabis platform applicable to solving multiple
industry challenges facing dispensaries and consumers. This would include
addressing issues such as transactional difficulties, inadequate product
labeling, product identification assurance and product origin assurance.”
IGC’s Product Portfolio
India Globalization Capital, Inc. (NYSE: IGC) is a first mover in the
dynamic cannabis market. The company has a portfolio of cannabis-based products
for a range of medical conditions, including Alzheimer’s and Parkinson’s
diseases, pain, nausea, eating disorders and epilepsy in cats and dogs.
As earlier noted, IGC has identified exceptional potential within the
Alzheimer’s market, which it is targeting with Hyalolex.
With an estimated cost to the economy of $236 billion, Alzheimer’s is America’s
most expensive disease. It currently affects over 5 million Americans, and this
is expected to double over the next 20 years. In collaboration with the
University of South Florida, IGC holds the exclusive license for the U.S. patent
filing entitled “THC as a Potential Therapeutic Agent for Alzheimer’s Disease.”
IGC also has combination therapy formulations for the treatment of epilepsy and
cachexia. Epilepsy affects around 50 million people globally, while 1.3 million
U.S. citizens suffer from cachexia associated with cancer, multiple sclerosis
(MS), Parkinson’s disease, HIV/AIDS and other progressive diseases. It is
reported that cancer-induced cachexia/anorexia accounts for 20 percent of all
cancer deaths. The company has two products in its development pipeline, IGC-502
indicated for seizures and IGC-504 indicated for cachexia. These are unique
combination therapies that, once proven in clinical trials, are expected to
exhibit reduced side effects as compared with conventional mono therapies used
in the treatment of medical refractory epilepsy and eating disorders.
Pain therapy presents a huge market opportunity. The national cost of treating
health issues related to pain is up to $635 billion, and a further $25 billion
is attributed to the health care cost of prescription opioid abuse. IGC has
filed a patent for a cannabis-based formulation, IGC-501, that uses a variety of
delivery techniques to treat neuropathic and arthritic pain in joints and
muscles. The company expects that this combination therapy, after completing
clinical trials, will provide a cannabinoid-based alternative to long-term
addictive opioid treatments.
IGC’s intention is to become a leader in the phytocannabinoid-based specialty
pharmaceutical sector, leveraging its first-mover advantage in combination
therapy development to build up its patent portfolio.
“The development of combination therapies utilizing cannabis represents a large,
unique opportunity in this emerging specialty-pharmaceutical sector. Securing
FDA approval for combination therapy is believed to be significantly faster and
less expensive than new drug applications. As a result, we believe that we can
bring our cannabis-based pharmaceutical products to market in both an
expeditious and cost-effective manner,” the company stated in a previous press
release.
Expanding its market beyond its product pipeline, IGC is entering the blockchain
market, where other companies are finding footing in a variety of applications.
Eastman Kodak Company (NYSE: KODK) recently announced a licensing partnership
with WENN Digital to revolutionize content rights management for photographers.
This initiative will utilize a secure blockchain platform to enable both amateur
and professional photographers to market their work in a space that will
generate more than $110 billion by 2021. The company will use its newly launched
KODAKOne, an encrypted management platform and digital ledger of rights
ownership, in conjunction with KODAKCoin, a photo-centric cryptocurrency, for
this venture.
BTL Group Ltd (OTC: BTLLF) is an established developer of blockchain
technologies that primarily serves the finance, energy and gaming industries.
The company’s secure, user-friendly and multi-chain platform, Interbit, enables
companies to build custom applications in trading, remittance, settlement, audit
and back-office processes. Another technology platform, Xapcash, in combination
with Interbit, will leverage blockchain technology for “cash-in, cash-out”
settlement solutions. BTL has collaborated with VISA Europe to initiate a pilot
program for the development of a cross-border settlement solution that
incorporates privacy, security and scalability.
Riot Blockchain (NASDAQ: RIOT) was originally known as Bioptrix Pharma, focusing
on biopharmaceuticals. The company experienced rapid growth after it reoriented
its focus to blockchain technology and its stock surged from $7 in November 2017
to more than $46 in a month. Riot Blockchain acquired Verady, LLC, a company
that provides accounting and auditing services to the digital currency market.
The company also owns a share in a Canadian-based cryptocurrency exchange,
Coinsquare. In addition, it owns a majority share in TessPay, a payment resource
for wholesale telecom carriers based on blockchain technology.
China Information Technology’s (NASDAQ: CNIT) integrated cloud-based solutions
enable innovation in several diverse fields, including education, new media and
elevator safety management. The company recently announced its intention to
research the application of blockchain for its Taoping new-media sharing
ecosystem to develop a more efficient and secure solution for payments and to
create a better mechanism for collecting internal data and rewarding end users.
Jianghuai Lin, chairman and CEO of CNIT said, “We believe that the research on
blockchain is a promising opportunity for us. As the developer and leading
operator of the Taoping ecosystem, we keep up with the development of new
technologies as we are devoted to keeping upgrading and optimizing our
services.”
While the volatility of cryptocurrencies keeps some skepticism afloat, an
increasing number of companies are developing innovative blockchain solutions
for their businesses. This technology is rapidly gaining acceptance for
widespread use in many industries as companies that explore its applications
find footing for potential long-term growth.
For more information on India Globalization Capital, visit
India Globalization Capital, Inc. (NYSE: IGC)
About NetworkNewsWire
NetworkNewsWire (NNW) is an information service that provides (1) access to our
news aggregation and syndication servers, (2) NetworkNewsBreaks that summarize
corporate news and information, (3) enhanced press release services from
NetworkWire, (4) social media distribution and optimization services, and (5) a
full array of corporate communication solutions. As a multifaceted financial
news and content distribution company with an extensive team of contributing
journalists and writers, NNW is uniquely positioned to best serve private and
public companies that desire to reach a wide audience of investors, consumers,
journalists and the general public. NNW has an ever-growing distribution network
of more than 5,000 key syndication outlets across the country. By cutting
through the overload of information in today’s market, NNW brings its clients
unparalleled visibility, recognition and brand awareness. NNW is where news,
content and information converge. For more information, please visit https://www.NetworkNewsWire.com
Please see full terms of use and disclaimers on the NetworkNewsWire website
applicable to all content provided by NNW, wherever published or re-published:
http://NNW.fm/Disclaimer
DISCLAIMER: NetworkNewsWire (NNW) is the source of the Article and content set
forth above. References to any issuer other than the profiled issuer are
intended solely to identify industry participants and do not constitute an
endorsement of any issuer and do not constitute a comparison to the profiled
issuer. The commentary, views and opinions expressed in this release by NNW are
solely those of NNW. Readers of this Article and content agree that they cannot
and will not seek to hold liable NNW for any investment decisions by their
readers or subscribers. NNW is a news dissemination and financial marketing
solutions provider and are NOT registered broker-dealers/analysts/investment
advisers, hold no investment licenses and may NOT sell, offer to sell or offer
to buy any security.
The Article and content related to the profiled company represent the personal
and subjective views of the Author, and are subject to change at any time
without notice. The information provided in the Article and the content has been
obtained from sources which the Author believes to be reliable. However, the
Author has not independently verified or otherwise investigated all such
information. None of the Author, NNW, or any of their respective affiliates,
guarantee the accuracy or completeness of any such information. This Article and
content are not, and should not be regarded as investment advice or as a
recommendation regarding any particular security or course of action; readers
are strongly urged to speak with their own investment advisor and review all of
the profiled issuer’s filings made with the Securities and Exchange Commission
before making any investment decisions and should understand the risks
associated with an investment in the profiled issuer’s securities, including,
but not limited to, the complete loss of your investment.
NNW HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains “forward-looking statements” within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. “Forward-looking statements” describe future expectations,
plans, results, or strategies and are generally preceded by words such as “may”,
“future”, “plan” or “planned”, “will” or “should”, “expected,” “anticipates”,
“draft”, “eventually” or “projected”. You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company’s annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW undertakes no obligation to update such
statements.
NetworkNewsWire (NNW) is affiliated with the Investor Brand Network (IBN).
About IBN
Over the past 10+ years we have consistently introduced new network brands, each
specifically designed to fulfil the unique needs of our growing client base and
services. Today, we continue to expand our branded network of highly influential
properties, leveraging the knowledge and energy of specialized teams of experts
to serve our increasingly diversified list of clients.
Please feel free to visit the Investor Brand Network (IBN)
www.InvestorBrandNetwork.com
Corporate Communications Contact:
NetworkNewsWire (NNW)
New York, New York
www.NetworkNewsWire.com
212.418.1217 Office
Editor@NetworkNewsWire.com
Media Contact:
FN Media Group, LLC
NNW@FinancialNewsMedia.com
+1-(954)345-0611
News Source: NetworkNewsWire
Leaders in Medical Marijuana and
the Global Alzheimer’s Markets
New York, NY – October 31, 2017 –
NetworkNewsWire.com News Coverage:
By now, the potential for cannabinoids like tetrahydrocannabinol (THC) and
cannabidiol (CBD) to reduce the symptoms and even help prevent the onset of
diseases such as Alzheimer’s has been fairly well-documented by a wide variety
of studies (http://nnw.fm/RRjS8). The global
 potential for cannabis-based
therapies has triggered a medical marijuana arms race, as countries like Canada,
Israel and many of those in Western Europe seize the initiative and more boldly
go where the U.S. federal government has feared to allow
private industry to tread. Rising stars in the sector, such as phytocannabinoid
combination therapy developer
India Globalization Capital, Inc. (NYSE: IGC), are acutely aware of this
immense global potential and have begun to position themselves to secure what
will no doubt be hotly-contested market share. Investors interested in medicinal
marijuana and/or Alzheimer’s treatments will find themselves looking at a global
landscape of key players that includes Medical Marijuana, Inc. (OTC: MJNA), the
first U.S. publicly traded cannabis company, as well as Canadian
pharmaceutical-grade marijuana producers Aphria, Inc. (OTC: APHQF) (TSX: APH),
MYM Nutraceuticals, Inc. (OTC: MYMMF) (CSE: MYM) and Supreme Pharmaceuticals,
Inc. (OTC: SPRWF) (TSX-V: FIRE). potential for cannabis-based
therapies has triggered a medical marijuana arms race, as countries like Canada,
Israel and many of those in Western Europe seize the initiative and more boldly
go where the U.S. federal government has feared to allow
private industry to tread. Rising stars in the sector, such as phytocannabinoid
combination therapy developer
India Globalization Capital, Inc. (NYSE: IGC), are acutely aware of this
immense global potential and have begun to position themselves to secure what
will no doubt be hotly-contested market share. Investors interested in medicinal
marijuana and/or Alzheimer’s treatments will find themselves looking at a global
landscape of key players that includes Medical Marijuana, Inc. (OTC: MJNA), the
first U.S. publicly traded cannabis company, as well as Canadian
pharmaceutical-grade marijuana producers Aphria, Inc. (OTC: APHQF) (TSX: APH),
MYM Nutraceuticals, Inc. (OTC: MYMMF) (CSE: MYM) and Supreme Pharmaceuticals,
Inc. (OTC: SPRWF) (TSX-V: FIRE).
As attitudes grow increasingly favorable to the medicinal capabilities of
cannabis, the companies that excel in this new field are those focused on the
development of therapies that address global market concerns, such as
Alzheimer’s. According to the Alzheimer’s Association, Alzheimer’s disease and
other dementias will cost the U.S. roughly $259 billion in 2017 and, by 2050,
could rise to as much as $1.1 trillion (http://nnw.fm/OzoX8).
Help may be on the way, though, as
India Globalization Capital, Inc. (NYSE: IGC) is now on the verge of
bringing a potential blockbuster treatment for Alzheimer’s to market as soon as
early 2018 (http://nnw.fm/8pkAv). Through a license agreement with the
University of South Florida (http://nnw.fm/Ny7N0), IGC is the exclusive licensee
of the U.S. patent filing “THC as a Potential Therapeutic Agent for Alzheimer’s
Disease,” putting the company on track with a unique advantage in the global
Alzheimer’s drug market, which is currently valued at roughly $3.6 billion
(http://nnw.fm/b6AVl).
Novel in vitro data (http://nnw.fm/wvwX7) aggregated by IGC using genetically
engineered cell lines add substantial weight to the existing argument about low
doses of THC addressing the link between amyloid beta peptide (Aβ plaque)
buildup in the cerebral cortex/hippocampus and Alzheimer’s (http://nnw.fm/lnxQ1).
Given that the dominant therapies for Alzheimer’s cannot stop or reverse disease
progression, the mounting evidence that THC and CBD can stop or even reverse the
symptoms of this disease (while providing neuroprotective benefits) is huge
(http://nnw.fm/f8RVr).
Today’s dominant Alzheimer’s therapies, such as Allergan’s (AGN) Namenda,
Daiichi Sankyo’s (DSNKY, DSKYF) Memary, Novartis’s (NVS) Exelon and the drug
Aricept developed by Pfizer (PFE) and Eisai (ESALY, ESALF), may soon find
themselves supplanted by disruptive new technologies based on extremely old
natural substances, like cannabis, which hav e been in continuous human use for
centuries. e been in continuous human use for
centuries.
Notably, while IGC’s potential is of impressive significance, the company’s
market valuation of $10 million is a drop in a bucket compared to other
Alzheimer’s players such as Allergan (NYSE: AGN), valued at $60+ billion;
Novartis (NYSE: NVS) at $213 billion, and Pfizer (NYSE: PFE) with a market cap
of over $210 billion. While IGC has considerable room to grow as investors
become aware of its true value, the company offers potentially the most
substantial product in the industry. If its aforementioned patent is approved,
IGC will own the key therapeutic pathway by which THC interacts with the human
body.
The new in vitro data from IGC extend earlier findings regarding the company’s
IGC-AD1 product (Hyalolex, http://nnw.fm/Xb0f4), which showed up to 50 percent
reduction in the production of the two key peptides that make up the amyloid
plaques found in abundance within the brains of Alzheimer’s patients (http://nnw.fm/o5PGh).
Moreover, because Alzheimer’s starts several decades before symptoms begin to
exhibit, a drug like IGC-AD1, which has demonstrated an ability to decrease
production/aggregation of Aβ plaque without neurotoxic effects or inebriation,
could become a leading prophylactic treatment taken by millions as a way to
prevent Alzheimer’s.
Building on this potential, IGC is gearing up to commercialize a non-inebriating
liquid supplement version of the product for the licensed medical dispensary
market (http://nnw.fm/4RgWz), which has demonstrated an ability to enhance
mitochondrial function (a trigger for Alzheimer’s pathophysiology) by as much as
60 percent in vitro (http://nnw.fm/0kf0N). This readily commercialized
combination therapy could give IGC significant, immediate ground game in this
burgeoning retail space and help support the company’s clinical work. This
one-two punch approach, with a food supplement in one hand and an FDA
prophylactic/therapeutic indication in the other, is as powerful as it is
solidly backed by exclusively-licensed IP.
Germany alone has nearly 83 million people, and the most common cause behind the
1.6 million-plus dementia cases in that country is Alzheimer’s (http://nnw.fm/Y1F5z).
As such, the recent announcement that IGC has entered into an MOU with leading
Hamburg-based medical cannabis information and services provider MediCann
Handels GmbH (http://nnw.fm/ssMn9) to import and distribute IGC’s cannabinoid-based
therapies to German pharmacies has been music to savvy investors’ ears. This is
a serious boon for IGC, which marks the first step of many along the company’s
path to commercialization and is a real sweetheart of a deal, with MediCann
footing the bill for the logistics, as well as sales and marketing.
Medical Marijuana (OTC: MJNA), with a current estimated market valuation of $67
million, is also eyeing its potential in the Alzheimer’s market, with its Real
Scientific Hemp Oil (“RSHO™”) products taking shape as leading CBD hemp oil
solutions in several international markets. In February 2017, MJNA announced
(http://nnw.fm/5B76p) that the Brazilian government authorized a doctor's
prescription for RSHO™ to treat a patient with Alzheimer’s disease.
"Treatment solutions for neurological diseases like Alzheimer's and Parkinson's
are desperately needed because of the difficulty in developing pharmaceuticals
that can pass the blood-brain barrier, a specialized system of cells that allow
nutrients in the brain while preventing harmful substances from passing
through," MJNA CEO Dr. Stuart Titus stated in the press release. "Cannabinoids
like CBD, however can pass through the blood-brain barrier and affect
cannabinoid/other receptors in the brain and an early stage study has shown the
potential benefit of cannabis and cannabinoid therapies for Alzheimer's disease.
This will lead us toward future cannabinoid remedies and research to convince
the medical community of potential benefits."
MJNA currently has three products approved for importation into Brazil, which
are subsidized by the government as prescription medications for chronic pain,
epilepsy, and Parkinson’s disease.
With mounting interest in the potential of cannabis-based therapies, the demand
for medicinal-grade cannabis production continues to increase. Canadian medical
cannabis provider Aphria (OTCQB: APHQF) (TSX: APH.TO)recently completed its
initial shipment of cannabis oil to Australian medical life science company
Medlab Clinical (http://nnw.fm/x0nFO). Aphira will produce and supply high-yield
cannabis extracts for Medlab’s upcoming, first-of-its-kind globally, human trial
of its cannabis-based NanaBis™ in intractable pain among oncology patients. With
product formulation and permits for the agreement already approved by both
Health Canada and the Australian Therapeutic Goods Administration, investors can
see why eyes are increasingly turning overseas amid the continued blossoming of
the cannabis sector into a full-fledged industry, with global horizons in the
ballpark of $56 billion by 2025 for just the non-recreational segment (http://nnw.fm/6Futo).
In Australia, MYM Nutraceuticals (OTCQB: MYMMF) (CSE: MYM.CN) is focused on
acquiring Health Canada licenses to produce and sell high-end organic medicinal
cannabis supplements and topical products. The company recently entered into a
strategic partnership with PUF Ventures, Inc. (CSE: PUF) (FRA: PU3) (OTC: PUFXF)
for the construction of a one million-square-foot greenhouse facility with
large-scale manufacturing, processing and office facilities for the cultivation,
production and manufacture of medical cannabis and associated products. At full
scale, the Northern Rivers Project will have the capacity to produce 100,000
kilograms of high quality cannabis per year, which would be worth between C$800
million and C$1.1 billion based on current pricing metrics in the Australian
cannabis marketplace (http://nnw.fm/nPNV9).
Supreme Pharmaceuticals (OTCQB: SPRWF) (TSX-V: FIRE.V). is dedicated to becoming
a leading cultivator and supplier of cannabis in federally legal and regulated
global markets. SPRWF is primed for access to global operators in the sector via
its wholly owned 7ACRES subsidiary, with sales already going swimmingly to other
Canadian players such as retail partner Aurora Cannabis (ACBFF) and medicines
developer Emerald Health Therapeutics (EMHTF). The company even recently tacked
on another 30,000 square feet at its (now) 342,000-square-foot hybrid greenhouse
facility in Ontario (http://nnw.fm/SuWN0).
With nearly 44 million people suffering from Alzheimer’s or a related dementia
worldwide and an estimated total health care cost of some $605 billion, this one
vector could carry much of the existing cannabis market, if a suitable THC/CBD-based
treatment is developed. Until many more U.S. states pass reform legislation or
the federal government reverses its stance on the drug, it seems reasonable that
market participants will continue to focus on global opportunities. As a
small-cap racing alongside large-cap peers, IGC is geared up to capture its
share of the medicinal marijuana and Alzheimer’s disease treatment markets,
propelled by potential patent protection for its market-ready technology, and
in-vitro, in-vivo clinical data showing the efficacy of its formulation.
For more information on India Globalization Capital please visit:
India Globalization Capital, Inc. (NYSE: IGC)
DISCLAIMER: NetworkNewsWire (NNW) is the source of the Article and content set
forth above. References to any issuer other than the profiled issuer are
intended solely to identify industry participants and do not constitute an
endorsement of any issuer and do not constitute a comparison to the profiled
issuer. FN Media Group (FNM) is a third-party publisher and news dissemination
service provider, which disseminates electronic information through multiple
online media channels. FNM is NOT affiliated with NNW or any company mentioned
herein. The commentary, views and opinions expressed in this release by NNW are
solely those of NNW and are not shared by and do not reflect in any manner the
views or opinions of FNM. Readers of this Article and content agree that they
cannot and will not seek to hold liable NNW and FNM for any investment decisions
by their readers or subscribers. NNW and FNM and their respective affiliated
companies are a news dissemination and financial marketing solutions provider
and are NOT registered broker-dealers/analysts/investment advisers, hold no
investment licenses and may NOT sell, offer to sell or offer to buy any
security.
The Article and content related to the profiled company represent the personal
and subjective views of the Author, and are subject to change at any time
without notice. The information provided in the Article and the content has been
obtained from sources which the Author believes to be reliable. However, the
Author has not independently verified or otherwise investigated all such
information. None of the Author, NNW, FNM, or any of their respective
affiliates, guarantee the accuracy or completeness of any such information. This
Article and content are not, and should not be regarded as investment advice or
as a recommendation regarding any particular security or course of action;
readers are strongly urged to speak with their own investment advisor and review
all of the profiled issuer's filings made with the Securities and Exchange
Commission before making any investment decisions and should understand the
risks associated with an investment in the profiled issuer's securities,
including, but not limited to, the complete loss of your investment.
NNW & FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW and FNM undertake no obligation to update
such statements.
NetworkNewsWire (NNW) is affiliated with the Investor Based Brand Network (IBBN).
About IBBN
Over the past 10+ years we have consistently introduced new network brands, each
specifically designed to fulfil the unique needs of our growing client base and
services. Today, we continue to expand our branded network of highly influential
properties, leveraging the knowledge and energy of specialized teams of experts
to serve our increasingly diversified list of clients.
Please feel free to visit the Investor Based Brand Network (IBBN)
www.InvestorBasedBrandNetwork.com
Corporate Communications Contact:
NetworkNewsWire (NNW)
New York, New York
www.NetworkNewsWire.com
212.418.1217 Office
Editor@NetworkNewsWire.com
Media Contact:
FN Media Group, LLC
NNW@FinancialNewsMedia.com
+1-(954)345-0611
News Source: NetworkNewsWire
_______________________________________________
Recent IGC News Coverage
IGC to Distribute its
Formulations in Germany in Early 2018
BETHESDA, Md., October 25, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
announced that it has entered into a Memorandum of Understanding (MOU) with
MediCann Handels GmbH, a company based in Hamburg Germany, for the import and
distribution of IGC’s cannabinoid based therapies including IGC-AD1 (Hyalolex)
to pharmacies in Germany.
MediCann plans to distribute to pharmacies, apothecaries and other licensed
retail outlets that are legally allowed to sell cannabinoid-based products.
Under the current MOU, MediCann will provide the capital required for the
transportation, import, storage, sales and marketing of products intended for
the German market. The agreement is subject to standard closing conditions such
as due diligence relating to satisfactory licensing and product procurement.
“There are many benefits to developing a strong presence in the German pharmacy
market including, a favorable regulatory environment and reimbursement from
health insurance providers. The agreement with MediCann is the first step in the
commercialization process of our therapies, as we work out the logistics of how
to efficiently deliver our products to Germany," states Ram Mukunda, CEO. “We are glad to add the products of IGC to
our portfolio. With these products we will have a unique selling point in the
German market,” states Carsten Siegemund, CEO of MediCann.
Germany," states Ram Mukunda, CEO. “We are glad to add the products of IGC to
our portfolio. With these products we will have a unique selling point in the
German market,” states Carsten Siegemund, CEO of MediCann.
About IGC
IGC is engaged in the development of cannabis based combination therapies to
treat Alzheimer’s, pain, nausea, eating disorders, several end points of
Parkinson’s, and epilepsy in dogs and cats. IGC has assembled a portfolio of
patent filings and four lead product candidates addressing these conditions. The
company is based in Maryland, USA.
For more information please visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see forward looking statements as discussed in detail in IGC's Form 10K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
Contact:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
IGC-AD1 Targeting Alzheimer’s
Disease to be commercialized in early 2018 Through Medical Dispensaries
IGC-AD1 for Alzheimer’s
Shows Promise by Inhibiting GSK3β and Tau hyperphosphorylation a key hallmark of
AD
BETHESDA, Md., October 18, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
provides compelling in vitro data compiled from genetically engineered cell
lines within an Alzheimer’s disease model, showing that at varying
concentrations of IGC-AD1 the expression of GSK3β is reduced by as much as 62%,
leading in turn to a reduction in hyper phosphorylation of tau protein.
“Based on this and other previously announced compelling data, we are readying
IGC-AD1, brand name Hyalolex, in a liquid formulation for commercialization in
early 2018,” stated IGC’s CEO, Ram Mukunda.
We have identified Germany, Canada and certain licensed medical cannabis states
in the U.S. for commercialization. The German market recently opened for imports
of cannabis products that can be sold in licensed pharmacies. Our initial
research indicates that there are about 7.8 million patients with AD in these
combined markets.
One of the two types of legions found in the brain of AD patients is
intracellular neurofibrillary tangles (NFTs) composed of tau protein. This study
shows that IGC-AD1 inhibits glycogen synthase kinase-3β (GSK3β) a major kinase
(catalyst) in the phosphorylation of tau protein. Curtailing abnormal
hyperphosphorylation of tau, which leads to NFTs, is an accepted strategy for combating AD.
strategy for combating AD.
Tau proteins are Microtubule Associated Proteins (MAPs) that stabilize
microtubules within a neuron. Abnormally phosphorylated tau leads to a
disassociation of tau from MAP, leading to a destabilization of microtubule
associated protein complexes; eventually leading to neuronal degeneration.
Studies have shown that in the brains of AD patients the phosphorylation of tau
is 3 to 4 times more than in normal brains.
This study result, when combined with the earlier reported data that shows
IGC-AD1 reduces Aβ production and inhibits Aβ aggregation without any neuronal
toxicity, represents a novel breakthrough. The summary in vitro data indicates
that at varying concentrations of IGC-AD1, GSK3β levels decreased between 53%
and 62%. This in turn curtailed hyperphosphorylation of tau protein as measured
by immunoblotting studies on N2aAβPPswe cells. Dr. Chuanhai Cao, IGC’s Senior
Advisor and Associate Professor of Pharmaceutical Sciences at USF’s College of
Pharmacy conducted the studies.
About Alzheimer’s Disease
Alzheimer’s Disease (AD) is a form of dementia. It is known as America’s most
expensive disease, with an estimated cost to the U.S. economy of $236 billion.
AD currently affects more than 5.3 million Americans and over 65% of AD patients
are women. Over the next 20 years, the number of those afflicted with the
disease is expected to double. The forecast is staggering, considering that to
date, no effective cure has been found.
About IGC
IGC is engaged in the development of cannabis based combination therapies to
treat Alzheimer’s, pain, nausea, eating disorders, several end points of
Parkinson’s, and epilepsy in dogs and cats. IGC has assembled a portfolio of
patent filings and four lead product candidates addressing these conditions. The
company is based in Maryland, USA.
For more information please visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see forward looking statements as discussed in detail in IGC's Form 10K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
Contact:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
IGC Prepares a Liquid Delivery
Formulation of IGC-AD1 for Alzheimer’s Based on Novel In Vitro Data
Readies Line of Medical
Dispensary Products Targeting Enhanced Mitochondrial Function in AD patients
BETHESDA, Md., October 4, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
provides an update on compelling in vitro data compiled from genetically
engineered cell lines within an Alzheimer’s Disease (AD) model, showing that at
varying concentrations of IGC-AD1 mitochondrial function is enhanced between 30%
and 60%.
“Based on our analysis of this and other previously announced compelling data,
we are readying IGC-AD1 in a liquid formulation that will not cause inebriation.
The initial commercialization will be through licensed medical cannabis
dispensaries,” stated IGC’s CEO, Ram Mukunda.
Mitochondrial dysfunction is thought to be at the apex of the AD pyramid, which
is believed to trigger a cascade leading to AD. Studies have indicated that
overproduction of Aβ disturbs the dynamics of mitochondrial fusion/fission,
among others, and alters mitochondrial function. It is theorized that age related
mitochondrial dysfunction leads to two types of legions found in the brains of
AD patients: senile plaques composed of amyloid beta proteins and
neurofibrillary tangles (NFTs) composed of tau protein.
and alters mitochondrial function. It is theorized that age related
mitochondrial dysfunction leads to two types of legions found in the brains of
AD patients: senile plaques composed of amyloid beta proteins and
neurofibrillary tangles (NFTs) composed of tau protein.
The summary in vitro data indicates that exposure over 36 hours to IGC-AD1 with
a dosage between 2.5nM and 25nM concentration improved mitochondrial function
between 30% and 60%, in the presence of FCCP, at the basal. Dr. Chuanhai Cao,
IGC’s Senior Advisor and Associate Professor of Pharmaceutical Sciences at USF’s
College of Pharmacy conducted the studies.
About Alzheimer’s Disease
Alzheimer’s Disease (AD) is a form of dementia. It is known as America’s most
expensive disease, with an estimated cost to the U.S. economy of $236 billion.
AD currently affects more than 5.3 million Americans and over 65% of AD patients
are women. Over the next 20 years, the number of those afflicted with the
disease is expected to double. The forecast is staggering, considering that to
date, no effective cure has been found.
About IGC
IGC is engaged in the development of cannabis based combination therapies to
treat Alzheimer’s, pain, nausea, eating disorders, several end points of
Parkinson’s, and epilepsy in dogs and cats. IGC has assembled a portfolio of
patent filings and four lead product candidates addressing these conditions. The
company is based in Maryland, USA.
For more information please visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see forward looking statements as discussed in detail in IGC's Form 10K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
Contact:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
IGC Readies Line of Medical
Dispensary Products Targeting Alzheimer’s Disease Based on Novel Data
Drug Candidate for
Alzheimer’s Shows Promise by Inhibiting Aβ Aggregation without Neuron Damage
BETHESDA, Md., Sept. 18, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
provides an update on compelling in vitro data compiled from genetically
engineered cell lines within an Alzheimer’s disease model, showing that at
varying concentrations of THC, the aggregation of Aβ protein decreases by as
much as 40%.
“As Alzheimer’s progresses, synaptic dysfunction and the death of neurons lead
to memory loss. These study results, when combined with the earlier reported
data that shows IGC-AD1 reduces Aβ40 and Aβ42 production by as much as 50%, and
40%, without any toxicity, represent a highly significant novel breakthrough
that could potentially bring much needed relief from this devastating disease,”
states IGC’s CEO Ram Mukunda.
It is believed that a primary cause of AD is the buildup of senile plaque
composed of amyloid beta peptides (Aβ plaque) in the cerebral cortex and
hippocampus. The key pathogenic event in the onset of AD is Aβ peptide monomers
aggregating into prefibrillar oligomers (dimers, trimmers, tetramers and
oligomers). As AD progresses Aβ oligomers directly cause synaptic dysfunction
and the death of neurons, consequently leading to a loss of memory.
“A drug that (i) decreases production of Aβ, (ii) inhibits Aβ aggregation into
oligomers, (iii) is not toxic to neurons, and (iv) does not cause inebriation (high), could be a powerful weapon against AD and the
prevention of AD. In vitro, our product demonstrates these critical factors and
we are pursuing a patent filing that protects this therapy.
does not cause inebriation (high), could be a powerful weapon against AD and the
prevention of AD. In vitro, our product demonstrates these critical factors and
we are pursuing a patent filing that protects this therapy.
AD starts 20 to 25 years before symptoms like memory loss are manifested.
Statistically, there is an almost 50% chance of individuals over 80 years
contracting AD and over 65% of AD patients are women. Based on the findings of
these studies, our plan is two-fold. First, we will position IGC-AD1 as a drug
that can be used both as a treatment for AD, and as a prophylactic treatment for
the prevention of AD. Second, we will commercialize a supplement version to be
sold as a medical dispensary product. This will allow our team to work through
the FDA approval process for IGC-AD1, while securing market share in the medical
dispensary segment. This is a very exciting time for all our shareholders and I
look forward to providing updates on our progress in combatting this global
disease,” concludes IGC’s CEO Ram Mukunda.
The summary in vitro data indicates that between 2.5nM and 25nM THC
concentration, the formation of Aβ1-42 trimers and tetramers in N2aAPP cells are
reduced by up to 40% as determined by both fluorescence and immuno blotting
assays. Dr. Chuanhai Cao, IGC’s Senior Advisor and Associate Professor of
Pharmaceutical Sciences at USF’s College of Pharmacy conducted the studies.
About Alzheimer’s Disease
Alzheimer’s Disease (AD) is a form of dementia. It is known as America’s most
expensive disease, with an estimated cost to the U.S. economy of $236 billion.
AD currently affects more than 5.3 million Americans and over 65% of AD patients
are women. Over the next 20 years, the number of those afflicted with the
disease is expected to double. The forecast is staggering, considering that to
date, no effective cure has been found.
About IGC
IGC is engaged in the development of cannabis based combination therapies to
treat Alzheimer’s, pain, nausea, eating disorders, several end points of
Parkinson’s, and epilepsy in dogs and cats. IGC has assembled a portfolio of
patent filings and four lead product candidates addressing these conditions. The
company is based in Maryland, USA.
For more information please visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see forward looking statements as discussed in detail in IGC's Form 10K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
Contact:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
Alzheimer’s Disease: Cannabis
Formulation Shows Promise
New York, NY – Sept. 12, 2017 –
NetworkNewsWire.com News Coverage:
Known as America’s most expensive disease, with an estimated cost to the U.S.
economy of $236 billion in 2016, Alzheimer’s disease (AD) affects more than 5.3
million
 Americans.
Over the next 20 years, the number of those afflicted with the disease is
expected to double. The forecast is staggering, considering that no effective
cure has been found, but the quest for one continues, with
India Globalization Capital, Inc. (NYSE: IGC), Anavex Life Sciences
(NASDAQ: AVXL), Axovant Sciences (NASDAQ: AXON), AC Immune Ltd. (NASDAQ: ACIU)
and Biogen, Inc. (NASDAQ: BIIB) all exploring a variety of approaches to uncover
the pathological pathways of this chronic neurodegenerative disease. Americans.
Over the next 20 years, the number of those afflicted with the disease is
expected to double. The forecast is staggering, considering that no effective
cure has been found, but the quest for one continues, with
India Globalization Capital, Inc. (NYSE: IGC), Anavex Life Sciences
(NASDAQ: AVXL), Axovant Sciences (NASDAQ: AXON), AC Immune Ltd. (NASDAQ: ACIU)
and Biogen, Inc. (NASDAQ: BIIB) all exploring a variety of approaches to uncover
the pathological pathways of this chronic neurodegenerative disease.
It is believed that Alzheimer’s disease is caused by two types of legions in the
cerebral cortex and hippocampus: senile plaque composed of the protein beta-amyloid
(Aβ plaque), and neurofibrillary tangle, composed of highly phosphorylated Tau
protein. The surface of neurons has a protein called APP that is sectioned by
enzymes to free up the Aβ protein that is then cleared by the body. In
Alzheimer’s patients, however, there is an imbalance whereby Aβ protein is not
regulated and builds up abnormally into insoluble fibroles, creating senile
plaques.
Currently,
India
Globalization Capital, Inc. (NYSE: IGC) is the only publicly
traded pharmaceutical cannabis stock that addresses Alzheimer’s disease, which
positions the company with a first-mover advantage in phytocannabinoid-based
combination therapy ( http://nnw.fm/0QDQz ).
The company’s drug candidate, IGC-ADI, works through a molecular pathway that
allows low doses of tetrahydrocannabinol (THC) to 1) inhibit Aβ protein
production, 2) inhibit Aβ protein aggregation, 3) reduce protein phosphorylation,
4) potentially restore mitochondria function, and 5) potentially redirect the
immune system.
IGC’s evidence supporting this theory is based on two studies done on tissue and
mice at the University of South Florida (USF). The USF study found 1) THC to be
effective at lowering Aβ levels in N2a/AβPPswe cells at extremely low
concentrations in a dose-dependent manner over a 48-hour period; 2) that THC
directly interacts with Aβ protein, thereby inhibiting aggregation; 3) that THC
was effective at lowering both total GSK-3β levels and phosphorylated GSK-3β in
a dose-dependent manner at low concentrations; and 4) that low doses of THC can
increase mitochondria function. These studies led to the filing of a patent by
USF entitled, “THC as a Potential Therapeutic Agent for Alzheimer’s Disease.”
IGC acquired the exclusive right to this patent filing and expects to advance
the technology and IGC-AD1 through medical trials.
The theory is further supported by a study conducted by researchers at the Salk
Institute of Biological Studies, who drew similar conclusions. In June 2016, the
Institute, in a news release ( http://nnw.fm/E5Ecm
) headlined “Cannabinoids remove plaque-forming Alzheimer’s proteins from brain
cells,” revealed that its scientists had found preliminary evidence “that tetrahydrocannabinol (THC) and other compounds found in marijuana can promote
the cellular removal of amyloid beta, a toxic protein associated with
Alzheimer’s disease.”
tetrahydrocannabinol (THC) and other compounds found in marijuana can promote
the cellular removal of amyloid beta, a toxic protein associated with
Alzheimer’s disease.”
A look at other approaches in the market emphasize the unique position occupied
by IGC, as well as the exciting potential of ongoing research. Anavex Life
Sciences (AVXL), for example, is focused on research aimed at treating more than
just the symptoms of Alzheimer’s. The company’s lead candidate, Anavex 2-73,
recently completed a phase 2a trial in patients with mild to moderate
Alzheimer's disease, showing a favorable safety and bioavailability profile and
dose response curve. Early preclinical studies generated a great deal of
excitement, because they indicated that Anavex 2-73 could potentially halt or
reverse the course of Alzheimer’s disease through restoration of the body's
homeostasis, according to a report (
http://nnw.fm/uN7LN ).
At Axovant Sciences (NASDAQ: AXON), an air of expectation is mounting as the
company anxiously awaits the outcome of a phase 3 trial, started in 2015, for
its lead Alzheimer’s candidate, intepirdine. Results are expected by the end of
September 2017. Axovant’s drug works in conjunction with another Alzheimer’s
drug currently in use, donepezil, and acts on the 5HT6 receptor as an
antagonist. While donepezil inhibits the loss of acetylcholine, a chemical in
the brain that transmits signals, intepirdine appears to increase the production
of that vital medium.
In August, AC Immune (NASDAQ: ACIU) announced the discovery of new antibodies
that target biomarkers other than Aβ and tau. A news release (
http://nnw.fm/WA9xS ) reports that “These
next-generation antibodies were discovered using the company's proprietary
SupraAntigen™ platform, which has already generated four products in clinical
development, including crenezumab partnered with Genentech/Roche in Phase 3 for
Alzheimer's.”
Biogen (NASDAQ: BIIB) has also joined the quest for an Alzheimer’s elixir. Its
experimental drug to treat the condition, aducanumab, is now in pivotal trials.
The company’s valuation soared by more than a billion dollars after analysts at
Goldman Sachs (NYSE: GS) added it to their Equity Conviction List, according to
the Boston Business Journal (http://nnw.fm/HlL7E).
These efforts to defeat Alzheimer’s are fueled by compelling incentives. Some
5.5 million Americans suffer from the malady, which kills more than breast
cancer and prostate cancer combined. The market potential of a remedy has been
estimated at over $5 billion, putting IGC at a sweet spot in the conjecture of
the medicinal applications of THC and disease treatment.
For more information on India Globalization Capital, please visit:
India
Globalization Capital, Inc. (NYSE: IGC) or
http://www.igcinc.us
About NetworkNewsWire
NetworkNewsWire (NNW) is an information service that provides (1) access to our
news aggregation and syndication servers, (2) NetworkNewsBreaks that summarize
corporate news and information, (3) enhanced press release services, (4) social
media distribution and optimization services, and (5) a full array of corporate
communication solutions. As a multifaceted financial news and content
distribution company with an extensive team of contributing journalists and
writers, NNW is uniquely positioned to best serve private and public companies
that desire to reach a wide audience of investors, consumers, journalists and
the general public. NNW has an ever-growing distribution network of more than
5,000 key syndication outlets across the country. By cutting through the
overload of information in today’s market, NNW brings its clients unparalleled
visibility, recognition and brand awareness. NNW is where news, content and
information converge.
NetworkNewsWire (NNW)
New York, New York
www.NetworkNewsWire.com
212.418.1217 Office
Editor@NetworkNewsWire.com
Please see full terms of use and disclaimers on the NetworkNewsWire website
applicable to all content provided by NNW, wherever published or re-published:
http://NNW.fm/Disclaimer
DISCLAIMER: NetworkNewsWire (NNW) is the source of the Article and content set
forth above. References to any issuer other than the profiled issuer are
intended solely to identify industry participants and do not constitute an
endorsement of any issuer and do not constitute a comparison to the profiled
issuer. FN Media Group (FNM) is a third-party publisher and news dissemination
service provider, which disseminates electronic information through multiple
online media channels. FNM is NOT affiliated with NNW or any company mentioned
herein. The commentary, views and opinions expressed in this release by NNW are
solely those of NNW and are not shared by and do not reflect in any manner the
views or opinions of FNM. Readers of this Article and content agree that they
cannot and will not seek to hold liable NNW and FNM for any investment decisions
by their readers or subscribers. NNW and FNM and their respective affiliated
companies are a news dissemination and financial marketing solutions provider
and are NOT registered broker-dealers/analysts/investment advisers, hold no
investment licenses and may NOT sell, offer to sell or offer to buy any
security.
The Article and content related to the profiled company represent the personal
and subjective views of the Author, and are subject to change at any time
without notice. The information provided in the Article and the content has been
obtained from sources which the Author believes to be reliable. However, the
Author has not independently verified or otherwise investigated all such
information. None of the Author, NNW, FNM, or any of their respective
affiliates, guarantee the accuracy or completeness of any such information. This
Article and content are not, and should not be regarded as investment advice or
as a recommendation regarding any particular security or course of action;
readers are strongly urged to speak with their own investment advisor and review
all of the profiled issuer's filings made with the Securities and Exchange
Commission before making any investment decisions and should understand the
risks associated with an investment in the profiled issuer's securities,
including, but not limited to, the complete loss of your investment.
NNW & FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW and FNM undertake no obligation to update
such statements.
Media Contact e-mail: FN Media Group, LLC - editor@financialnewsmedia.com
(954)345-0611
News Source: NetworkNewsWire
IGC’s Drug Candidate for
Alzheimer’s Potentially Presents New Molecular Pathway for THC to Inhibit Aβ40
Production
BETHESDA, Md., Sept. 11, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
today provides an update on compelling in vitro data compiled from genetically
engineered cell lines, confirmed by Dr. Chuanhai Cao, an IGC Senior Advisor and
Associate Professor of Pharmaceutical Sciences at USF’s College of Pharmacy,
which shows that at varying concentrations of THC, the production of Aβ40
protein decreases by as much as 50% over a 48-hour period.
It is believed that Alzheimer’s disease is caused by two types of legions in the
cerebral cortex and hippocampus: 1) senile plaque composed of amyloid beta
peptides (Aβ plaque), and 2) neurofibrillary tangle, composed of highly
phosphorylated Tau protein. Amyloid Protein Precursor (APP), on the surface of
neurons, is normally cleaved by enzymes to free up Aβ peptide composed of 36-43
amino acids that is then cleared by the body.
In patients with Alzheimer’s, an imbalance causes Aβ to be unregulated,
resulting in the abnormal buildup into insoluble fibroles depositing as senile
plaques. Aβ monomers aggregate to form oligomers and then into fibril Aβ. It is
believed that extracellular misfolded oligomers are toxic to nerve cells.
IGC’s Alzheimer’s drug candidate, IGC-ADI, works through a molecular pathway
that allows low doses of THC to: 1) inhibit Aβ protein production, 2) inhibit Aβ
protein aggregation 3) reduce protein phosphorylation, 4) restore mitochondria
function and 5) redirect the immune system.
and 5) redirect the immune system.
The summary data indicates that at a 25nM THC concentration, Aβ40 production
decreased by 30% over a 6-hour period; 35% over a 24-hour period; and 40% over a
48-hour period. At a 2.5 μM concentration of THC, Aβ40 production decreased by
30% over a 6-hour period; 40% over a 24-hour period; and 55% over a 48-hour
period.
Aβ40 and Aβ42 play a key role in amyloid plaques and have been implicated in the
pathogenesis of Alzheimer’s disease. The studies done by Dr. Cao at USF led to
the filing of a patent by USF entitled, “THC as a Potential Therapeutic Agent
for Alzheimer’s Disease.”
“IGC acquired the exclusive right to this patent filing and we plan to advance
this technology through medical trials that can potentially bring much needed
relief for patients suffering from AD,” says IGC’s CEO, Ram Mukunda.
About Alzheimer’s Disease
Alzheimer’s Disease (AD) is a form of dementia. It is known as America’s most
expensive disease, with an estimated cost to the U.S. economy of $236 billion.
AD currently affects more than 5.3 million Americans. Over the next 20 years,
the number of those afflicted with the disease is expected to double. The
forecast is staggering, considering that to date, no effective cure has been
found.
About IGC
IGC is engaged in the development of cannabis based combination therapies to
treat Alzheimer’s, pain, nausea, eating disorders, several end points of
Parkinson’s, and epilepsy in dogs and cats. IGC has assembled a portfolio of
patent filings and four lead product candidates addressing these conditions. The
company is based in Maryland, USA.
For more information please visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see forward looking statements as discussed in detail in IGC's Form 10K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
Investors Contact:
Claudia Grimaldi
301-983-0998
www.igcinc.us
info@igcinc.us
Source: India Globalization Capital
IGC Announces Financial
Results for Quarter Ended June 30, 2017
Alzheimer’s Licensing Agreement Highlights Cannabis-Based
IP Portfolio Targeting Large Market Conditions
BETHESDA, Md., August 21, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
announces financial results for the quarter ended June 30, 2017.
“During the quarter, we secured an IP licensing agreement from the University of
South Florida addressing a potential treatment for Alzheimer’s disease. This
patent filing claims discovery of a new pathway: low doses of THC bind to
amyloid beta plaques and prevent those plaques from aggregating on neurons,
which is what occurs in Alzheimer’s disease and causes cognitive decline. IGC
expects to release mouse data showing that this pathway and therapy have
possible blockbuster potential in treating Alzheimer’s disease. We expect to
take this exciting treatment to human medical trials as soon as possible,”
stated Ram Mukunda, CEO of IGC.
Total revenue was $52,926 for the three months ended June 30, 2017, as compared
to $288,493 for the three months ended June 30, 2016. The decrease in revenue is
attributable to the corporate mandate to extricate IGC from the electronic and
iron ore trading businesses and focus management on medical cannabis therapies.
The revenue in the three months ended June 30, 2017 is all from the rental of
heavy equipment and real estate management.
Selling, general and administrative expenses were $436,351 for the quarter ended
June 30, 2017 as compared to $307,772 for the quarter ended June 30, 2016. Most
of these expenses are a result of public-company expenses, including legal fees.
The Company reported a consolidated GAAP net income loss of $432,141 and a GAAP
EPS loss of $0.02 compared to a GAAP net income loss of $383,566 and a GAAP EPS
loss of $0.02 for the three months ended June 30, 2016. The increase in loss is
due to increased SG&A related to marketing programs that the company initiated.
For the quarter ended June 30, 2017, our non-GAAP cash burn was approximately
$389,577.
At the end of June 30, 2017, our cash and cash equivalents along with restricted
cash was $345,610 with working capital of $623,984. We anticipate securing
additional capital to further our patent portfolio and commence medical trials
on our Alzheimer’s product.
About IGC
IGC develops cannabis-based combination therapies to treat Alzheimer’s, pain,
nausea, eating disorders, several end points of Parkinson’s, and epilepsy in
humans, dogs and cats. In support of this effort, IGC has assembled a portfolio
of patent filings and four lead product candidates addressing these conditions.
We are based in Bethesda, Maryland.
Our website: www.igcinc.us. Twitter @IGCIR Facebook.com/IGCIR/
Forward-looking Statements
Please see forward-looking statements as discussed in detail in IGC's Form 10-K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
FINANCIAL STATEMENTS TO FOLLOW
INDIA GLOBALIZATION CAPITAL, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(All amounts in USD, except number of shares and per share amounts)
As of
30-June-17 31-Mar-17
(unaudited) (audited)
ASSETS
Current assets:
Cash and cash equivalents $ 345,610 $ 538,029
Accounts receivable, net of allowances 839,462 752,926
Prepaid expenses and other current assets 416,160 410,408
Short-term investments - 1,880,000
Total current assets $ 1,601,232 $ 3,581,363
Goodwill 198,169 198,169
Property, plant and equipment, net 952,009 953,936
Investments in affiliates 773,111 773,111
Investments-others 5,239,778 5,238,003
Other non-current assets 672,142 539,720
Total long-term assets $ 7,835,209 $ 7,702,939
Total assets $ 9,436,441 $ 11,284,302
LIABILITIES AND STOCKHOLDERS' EQUITY
Current liabilities:
Trade payables 427,079 416,532
Accrued expenses 143,000 181,465
Other current liabilities 407,169 691,714
Total current liabilities $ 977,248 $ 1,289,711
Long -term borrowings 175,991 452,080
Loans – others 388,476 392,226
Notes payable 1,800,000 1,800,000
Total non-current liabilities $ 2,364,467 $ 2,644,306
Total liabilities $ 3,341,715 $ 3,934,017
Stockholders' equity:
Common stock — $.0001 par value; 150,000,000 shares authorized; 23,265,531
issued and outstanding as of March 31, 2017 and 26,803,020 issued and
outstanding as of June 30, 2017. $ 2,680 $ 2,827
Additional paid-in capital 60,588,461 61,413,533
Accumulated other comprehensive income (2,046,023 ) (2,047,780 )
Retained earnings (Deficit) (52,441,602 ) (52,009,459 )
Total equity attributable to Parent $ 6,103,516 $ 7,359,121
Non-controlling interest $ (8,790 ) $ (8,836 )
Total stockholders' equity $ 6,094,726 $ 7,350,285
Total liabilities and stockholders' equity $ 9,436,441 $ 11,284,302
See accompanying Notes to Consolidated Financial Statements below in this report
and Notes to the Audited Consolidated Financial Statements contained in the
Company’s Annual Report on Form 10-K for the fiscal year ended March 31, 2017
filed with the SEC on July 14, 2017.
INDIA GLOBALIZATION CAPITAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(Unaudited)
(All amounts in USD, except number of shares and per share amounts)
As of
Three months ended June 30,
2017 2016
Revenues $ 52,926 $ 288,493
Cost of revenues (excluding depreciation) (6,880 ) (201,854 )
Selling, general and administrative expenses (436,351 ) (307,772 )
Depreciation (5,964 ) (97,672 )
Operating income (loss) $ (396,269 ) $ (318,805 )
Interest expense (44,546 ) (43,278 )
Other income, net 8,355 1,755
Income before income taxes and minority interest attributable to non-controlling
interest $ (432,460 ) $ (360,328 )
Income taxes benefit/ (expense) - -
Net income/(loss) $ (432,460 ) $ (360,328 )
Non-controlling interests in earnings of subsidiaries (319 ) 23,238
Net income / (loss) attributable to common stockholders $ (432,141 ) $ (383,566
)
Earnings/(loss) per share attributable to common stockholders:
Basic $ (0.02 ) $ (0.02 )
Diluted $ (0.02 ) $ (0.02 )
Weighted-average number of shares used in computing earnings per share amounts:
Basic 25,865,307 23,312,056
Diluted 25,865,307 23,312,056
See accompanying Notes to Consolidated Financial Statements below in this report
and Notes to the Audited Consolidated Financial Statements contained in the
Company’s Annual Report on Form 10-K for the fiscal year ended March 31, 2017
filed with the SEC on July 14, 2017.
Contact
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
IGC’s CEO and Key Scientific
Advisor Quoted in “HIGH TIMES” Article on THC and Alzheimer’s Disease
BETHESDA, Md., August 3, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
is pleased to announce the publication of “Marijuana Could Help Treat
Alzheimer’s: Here’s How It Would Work” on August 1, 2017 by Javier Hasse on the
website of “HIGH TIMES.”
IGC’s CEO, Ram Mukunda and IGC’s key advisor, Dr. Chuanhai Cao were quoted with
respect to the potential therapeutic effects of THC on Alzheimer’s Disease.
“In Alzheimer’s Disease, beta-amyloid aggregates into a plaque-like substance
that builds around the neurons and disrupts communication between them. So of
course, if low-doses of THC can break up those plaques and prevent them from
forming in the first place, it’s a huge breakthrough,” stated Ram Mukunda, CEO.
“The paper argued that therapeutic, low [non-psychoactive] doses of THC (one of
the main chemical compounds present in marijuana) could directly bind to a
protein called amyloid-beta, preventing its aggregation and thereby slowing down
the formation of amyloid-beta plaques around neurons,” Dr. Chuanhai Cao, a
professor of neurology and pharmacy at the University of South Florida’s Byrd Institute for Alzheimer’s.
University of South Florida’s Byrd Institute for Alzheimer’s.
The associated link for the full article can be found at: http://hightimes.com/medicinal/marijuana-could-help-treat-alzheimers-heres-how-it-would-work/
As previously reported, IGC entered into a definitive license agreement with the
University of South Florida making IGC the exclusive licensee of the U.S. patent
filing entitled “THC as a Potential Therapeutic Agent for Alzheimer’s Disease.”
By acquiring this patent filing, IGC is protecting a potential cannabis-based
blockbuster treatment for America’s most expensive disease.
About HIGH TIMES
HIGH TIMES is the definitive resource for all things cannabis. From cultivation
and legalization, to entertainment and culture, to hard-hitting news exposing
the War on Drugs, HIGH TIMES has been the preeminent source for cannabis
information since 1974.
About IGC
IGC is engaged in the development of cannabis based combination therapies to
treat Alzheimer’s, pain, nausea, eating disorders, several end points of
Parkinson’s, and epilepsy in humans, dogs and cats. In support of this effort,
IGC has assembled a portfolio of patent filings and four lead product candidates
addressing these conditions. The company is based in Maryland, USA.
For more information please visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see forward looking statements as discussed in detail in IGC's Form 10K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
Contact:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
IGC Builds Team Adding
Industry Leader Dr. Chuanhai Cao, an expert on THC-Based Alzheimer’s Disease
Treatments
BETHESDA, Md., August 1, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
is pleased to announce that Dr. Chuanhai Cao has joined its medical research
team as a key Advisor.
Dr. Cao is Associate Professor of Pharmaceutical Sciences, at the University of
South Florida’s (USF) College of Pharmacy. He also has joint appointments as
Associate Professor at USF’s department of Neurology at the College of Medicine,
and the department of CMMB at the College of Arts and Sciences.
“The addition of Dr. Cao will accelerate IGC’s efforts to move its Alzheimer’s
product Hyalolex to clinical trials. Dr. Cao is a dynamic force in cannabis
related therapies for Alzheimer’s disease. Dr. Cao has one co-inventor patent
that has been approved for medical trials, and another patent that is currently
being worked on for an Investigational New Drug (IND) application with the FDA.
Dr. Cao is the perfect researcher to assist us in moving our THC-based
Alzheimer’s treatment into trials and potentially into a blockbuster product.
The addition of Dr. Cao to the core group of medical and science advisors
consisting of Dr. Craig Cheifetz, Dr. Ranga Krishna and Dr. James Saunders
greatly strengthens our team as we seek to move our four products towards
commercialization,” concludes Ram Mukunda, CEO.
Dr. Cao conducted the research underlying USF’s patent filing for the use of THC
as a potential therapeutic agent for Alzheimer’s. The lab experiments using an
animal model led to the publication of findings that THC has the potential to
inhibit amyloid beta peptide aggregation and possibly restore memory function,
halting the progression of Alzheimer’s disease. IGC recently acquired exclusive
rights to this patent filing from USF.
Dr. Cao has a PhD, in Medical Microbiology and Virology, from Tianjin Medical
University, China. He has authored or co-authored around 80 peer-reviewed
articles. He has been quoted in Huffington Post, USA Today, and was featured in
the CNN documentary WEED 3 The Marijuana Revolution by Dr. Sanjay Gupta. Dr.
Cao’s interview can be viewed at https://vimeo.com/137431143#t=1818s.
“I became particularly interested in Alzheimer’s disease, especially after the
diagnoses of a few individuals close to me. In 2001, led by co-authors,
including well-respected Alzheimer’s pathology researchers at USF, I contributed
to my (then) first published paper on responses in experimental mice to a
vaccination for the Alzheimer’s disease-associated beta amyloid 1-42 peptide. I
would then go on to co-author over 10 papers studying beta amyloid in
Alzheimer’s mice, with a focus on potential treatments. In 2008, I helped to
first-author two papers reporting on potential vaccines for Alzheimer’s disease.
The first described the studying and adjuvant-free vaccination using mutated
amyloid beta peptides, and the second tested mutant amyloid-beta-sensitized
dendritic cells as a possible vaccine. I was also head of a patent on amyloid
beta peptides and methods of use. In 2012, I first-authored a book chapter on
amyloid beta vaccination strategies, and am currently pursuing a patent on
immunomodulatory cells as a novel treatment for diseases, in addition to
researching the use of cannabinoids for preventing amyloid beta aggregation.”
stated Dr. Cao.
About IGC
IGC is engaged in the development of cannabis based combination therapies to
treat Alzheimer’s, pain, nausea, eating disorders, several end points of
Parkinson’s, and epilepsy in humans, dogs and cats. In support of this effort,
IGC has assembled a portfolio of patent filings and four lead product candidates
addressing these conditions. The company is based in Maryland, USA.
For more information please visit www.igcinc.us.
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/.
Forward-looking Statements
Please see forward looking statements as discussed in detail in IGC's Form 10 K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
Contact:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
Potential Blockbuster Treatment for Alzheimer’s Disease Stands Out Among Medical Cannabis Companies
New York, NY – July 25, 2017 –
NetworkNewsWire.com News Coverage:
Changes in public opinion regarding cannabis have greatly fueled the fire
blazing under the red-hot state-legalized marijuana market. A recent Gallup poll
shows that 60
 percent
of Americans now support the legalization of marijuana1, while another report
shows that marijuana sales jumped an incredible 30 percent in North America in
2016 to reach $6.7 billion2. While many investors are looking to find profitable
plays in this burgeoning market, continuing federal restrictions make medical
marijuana one of the safest investment routes. Standout companies that present
prime opportunities in this space include
India Globalization Capital, Inc. (NYSE: IGC), Cara Therapeutics, Inc.
(NASDAQ: CARA), Zynerba Pharmaceuticals, Inc. (NASDAQ: ZYNE), Cannabis Science
Inc. (OTC: CBIS) and Vitality Biopharma, Inc. (OTC: VBIO). percent
of Americans now support the legalization of marijuana1, while another report
shows that marijuana sales jumped an incredible 30 percent in North America in
2016 to reach $6.7 billion2. While many investors are looking to find profitable
plays in this burgeoning market, continuing federal restrictions make medical
marijuana one of the safest investment routes. Standout companies that present
prime opportunities in this space include
India Globalization Capital, Inc. (NYSE: IGC), Cara Therapeutics, Inc.
(NASDAQ: CARA), Zynerba Pharmaceuticals, Inc. (NASDAQ: ZYNE), Cannabis Science
Inc. (OTC: CBIS) and Vitality Biopharma, Inc. (OTC: VBIO).
India Globalization Capital, Inc. (NYSE: IGC) is currently readying four
cannabinoid products for medical trials, including a potential blockbuster
treatment for Alzheimer’s disease. Although there are about a dozen publicly
traded cannabis pharmaceutical stocks currently on the market, IGC is the only
one to have patent filings for a potential cannabis-based Alzheimer’s
breakthrough. Compared to its peers, IGC’s market cap of just over $10.8 million
begs the question of whether the value of this innovator is under pegged. A look
at the broader market and the company’s developments provide further insight.
Alzheimer’s, the most common cause of dementia among older adults is a chronic
neurodegenerative disease that progressively destroys memory and cognitive
skills. Alzheimer’s is the sixth-leading cause of death in the United States and
will cost the nation an estimated $259 billion in 2017, according to information
from the Alzheimer’s Association3. The breakthrough potential of IGC’s IGC-AD1 (Hyalolex), which targets the reduction
of beta-amyloid buildup in Alzheimer’s patients and lessen some of Alzheimer’s
worst symptoms, could be astronomical.
breakthrough potential of IGC’s IGC-AD1 (Hyalolex), which targets the reduction
of beta-amyloid buildup in Alzheimer’s patients and lessen some of Alzheimer’s
worst symptoms, could be astronomical.
The accumulation of amyloid plaque on neurons in the brain is believed to be the
major cause of Alzheimer’s disease. IGC recently acquired exclusive rights
(http://nnw.fm/3QaWV) to its novel tetrahydrocannabinol (THC)-based treatment
for Alzheimer’s from the University of South Florida, where research using an
animal model showed the reversal of amyloid plaque buildup. The lead
investigator at the university used cannabis extract on transgenic mice and
discovered that the cannabis extract actually reversed beta-amyloid
accumulation. What this indicates is a potential means of restoring memory
function in Alzheimer’s patients—a game-changing development indeed! IGC expects
to begin human trials this year.
Under the definitive license agreement with the University of South Florida, IGC
is the exclusive licensee of the patent filing “THC as a Potential Therapeutic
Agent for Alzheimer’s Disease,” which claims the discovery of a new pathway in
which low doses of THC bind to beta-amyloid plaques and prevent them from
aggregating on neurons—the very process that is linked to causing cognitive
decline in Alzheimer’s patients. This new pathway offers exciting potential in
the treatment of Alzheimer’s, and, if the patent is granted and proven, IGC will
own the rights to it. Acquisition of the patent additionally helps IGC protect
its proprietary formulation of IGC-AD1. The lead investigator at the University
of South Florida now helping IGC was featured on CNN by Dr. Sanjay Gupta on the
“Weed 3, The Marijuana Revolution,” available for viewing at www.igcinc.us
In addition to IGC-AD1, the company’s other cannabis-based products currently
moving toward trials include Natrinol for the treatment of cachexia in AIDS and
cancer patients; Serosapse, which addresses various Parkinson’s disease
endpoints; and Caesafin, which employs combination therapy to alleviate seizures
in dogs and cats. Other companies we have seen with this kind of cannabis based
runaway potential, have market capitalizations between $25 million to $500
million as compared to IGC’s $10 million market cap.
When it comes to market value, the heavyweight of this group is clinical-stage
biopharmaceutical company Cara Therapeutics, Inc. (NASDAQ: CARA), which has a
market cap of $506.1 million. Focused on the development and commercialization
of new chemical entities that are designed to alleviate pain and pruritus
through the selective targeting of peripheral kappa opioid receptors, the
company is developing a novel, proprietary class of product candidates that
target the peripheral nervous system and have shown initial efficacy in patients
with moderate to severe pain without creating many of the unwanted side effects
that are typically associated with current pain therapeutics. The company’s
CR701 candidate is a cannabinoid receptor agonist designed to reduce pain.
Perhaps most similar to IGC in terms of product candidates and an early-stage
pipeline is Zynerba Pharmaceuticals, Inc. (NASDAQ: ZYNE), a clinical-stage
pharmaceutical company engaged in developing innovative transdermal synthetic
cannabinoid treatment for patients who have high unmet medical needs. As of July
18, Zynerba’s market cap stood at $251.7 million – a far cry above IGC’s market
valuation. Zynerba’s development pipeline includes two lead product candidates
that are being evaluated in five therapeutic indications. ZYN002 is the first
and only synthetic CBD and has been formulated as a patent-protected permeation
enhanced gel for transdermal delivery through the skin and into the body’s
circulatory system. It is currently in phase 2 clinical development in patients
with refractory epilepsy, osteoarthritis of the knee and Fragile X syndrome. The
company’s ZYN001 is a pro-drug of THC that facilitates transdermal delivery
through the skin and into the circulatory system by means of a patch. A phase 1
clinical program for ZYN001 commenced in the first half of 2017.
Another small-cap player – with a market cap of $120.2 million - is Cannabis
Science, Inc. (OTC: CBIS), a company focused on developing cannabinoid-based
medicines to provide innovative treatment options for unmet medical needs, with
an immediate focus on treating cancer. The company is working with leading
experts to develop, produce and commercialize novel therapeutic approaches to
treat a variety of critical ailments, from cancer and infections to age-related
illnesses and neurobehavioral disorders. The company’s current development
programs include CS-TATI-1, which will be targeted at both newly diagnosed and
treatment-experienced patients with drug-resistant HIV strains and those that
are intolerant to current therapies; CS-S/BCC-1 for skin cancer; and CS-NEURO-1
for various neurobehavioral disorders, including ADHD and anxiety.
Vitality Biopharma, Inc. (OTCQB: VBIO), another medical marijuana company, is
focused on applying the power of cannabinoids to treat serious neurological and
inflammatory disorders and currently has a market cap of $40.2 million. VBIO is
developing proprietary cannabinoid prodrug pharmaceuticals that are FDA- and
DEA-compliant. The company believes oral cannabinoid pharmaceuticals can help
the full therapeutic potential of cannabinoid medicines to be realized. Oral
prodrugs allow for a regulatory strategy that has a lower risk and is similar to
specialty pharmaceutical development. VBIO is leveraging existing clinical
studies that demonstrate the efficacy of cannabinoids in treating inflammatory
bowel disease, Nijmegen breakage syndrome (NBS), multiple sclerosis, neuropathic
pain and other disease indications. The company has developed a new class of
cannabinoid prodrugs called cannabosides that can be targeted and limited to the
body’s gastrointestinal tract to prevent drug psychoactivity and unanticipated
side effects.
The spectrum of market value for these cannabis-based pharmaceutical players’
swings across the board, and India Globalization Capital has several aspects
that may warrant a higher valuation. While each of the above-named companies
present an opportunity to invest in the rapidly advancing medical cannabis
space, investors looking for an entry point through an undervalued company may
want to take a closer look at India Globalization Capital, Inc. (NYSE: IGC).
Editorial Sources:
(1) Gallup http://nnw.fm/CPS7h
(2) Arcview http://nnw.fm/zE44Q
(3) Alzheimer’s Association http://nnw.fm/LpC8P
For more information on India Globalization Capital, please visit: IIndia
Globalization Capital, Inc. (NYSE: IGC) or
http://www.igcinc.us
About NetworkNewsWire
NetworkNewsWire (NNW) is an information service that provides to users (1)
access to our news aggregation and syndication servers, (2) enhanced press
release services, and (3) a full array of social communication solutions. As a
multifaceted financial news and content distribution company with an extensive
team of contributing journalists and writers, NNW is uniquely positioned to best
serve private and public companies that desire to reach a wide audience of
investors, consumers, journalists and the general public. NNW has an
ever-growing distribution network of more than 5,000 key syndication outlets
across the country. By cutting through the overload of information in today's
market, NNW brings its clients unparalleled visibility, recognition and brand
awareness. NNW is where news, content and information converge.
NetworkNewsWire (NNW)
New York, New York
www.NetworkNewsWire.com
212.418.1217 Office
Editor@NetworkNewsWire.com
Please see full terms of use and disclaimers on the NetworkNewsWire website
applicable to all content provided by NNW, wherever published or re-published:
http://NNW.fm/Disclaimer
DISCLAIMER: NetworkNewsWire (NNW) is the source of the Article and content set
forth above. References to any issuer other than the profiled issuer are
intended solely to identify industry participants and do not constitute an
endorsement of any issuer and do not constitute a comparison to the profiled
issuer. FN Media Group (FNM) is a third-party publisher and news dissemination
service provider, which disseminates electronic information through multiple
online media channels. FNM is NOT affiliated with NNW or any company mentioned
herein. The commentary, views and opinions expressed in this release by NNW are
solely those of NNW and are not shared by and do not reflect in any manner the
views or opinions of FNM. Readers of this Article and content agree that they
cannot and will not seek to hold liable NNW and FNM for any investment decisions
by their readers or subscribers. NNW and FNM and their respective affiliated
companies are a news dissemination and financial marketing solutions provider
and are NOT registered broker-dealers/analysts/investment advisers, hold no
investment licenses and may NOT sell, offer to sell or offer to buy any
security.
The Article and content related to the profiled company represent the personal
and subjective views of the Author, and are subject to change at any time
without notice. The information provided in the Article and the content has been
obtained from sources which the Author believes to be reliable. However, the
Author has not independently verified or otherwise investigated all such
information. None of the Author, NNW, FNM, or any of their respective
affiliates, guarantee the accuracy or completeness of any such information. This
Article and content are not, and should not be regarded as investment advice or
as a recommendation regarding any particular security or course of action;
readers are strongly urged to speak with their own investment advisor and review
all of the profiled issuer's filings made with the Securities and Exchange
Commission before making any investment decisions and should understand the
risks associated with an investment in the profiled issuer's securities,
including, but not limited to, the complete loss of your investment.
NNW & FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW and FNM undertake no obligation to update
such statements.
Media Contact e-mail: FN Media Group, LLC - editor@financialnewsmedia.com
(954)345-0611
News Source: NetworkNewsWire
IGC Announces Financial
Results for Fiscal Year Ended March 31, 2017
Core Operations Transitioned to Phytocannabinoid-Based Therapy Development
Targeting Large Market Indications
BETHESDA, Md., July 14, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
announces financial results for the fiscal year ended March 31, 2017.
“In fiscal 2017, our major accomplishments include the advancement of our
phytocannabinoid patent filing portfolio to large market indications. And in
order to keep this focus on the medical cannabis industry, we disposed of our
low-margin iron ore and electronic trading businesses, and retired about 10% of
our outstanding common stock; thus reducing revenue, PP&E, and stockholder’s
equity. We firmly believe that this planned strategic move positions our Company
for growth in one of the fastest growing industries in America,” said Ram
Mukunda, CEO.
Total revenue was approximately $0.58 million for FYE 2017, as compared to
approximately $6.37 million for the FYE 2016. Exiting the electronic business
contributed to the decrease in revenue.

As a result of our decision to exit the iron ore business segment, our FYE 2017
Property, Plant and Equipment, net of depreciation decreased by approximately
$6.1 million to approximately $0.95 million, and that also largely led to the
decrease in stockholder’s equity to about $7.3 million from about $13.9 million
in fiscal 2016.
Selling, general and administrative expenses were about $1.88 million for fiscal
2017, inclusive of one time expenses associated with the disposition of
businesses lines, and non-cash expenses, as compared to about $2.70 million for
fiscal 2016, an improvement of 30.6%. Fiscal 2017 reflects a steep cut in
expenses associated with a further aligning of resources to focus on
phytocannabinoid therapies.
Loss from operations was approximately $2.05 million in fiscal year 2017, as
compared to approximately $2.91 million in fiscal year 2016. The improvement in
operating loss year over year is mostly attributed to lower SG&A. At the end of
fiscal year 2017, the Company has approximately $0.54 million in cash and cash
equivalents and working capital of approximately $2.30 million.
Financial Tables to Follow
INDIA GLOBALIZATION CAPITAL, INC. AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(Audited)
(All amounts in USD, except number of shares and per share amounts)
31-March-17 31-March-16
(audited) (audited)
ASSETS
Current assets:
Cash and cash equivalents $ 538,029 $ 1,490,693
Accounts receivable, net of allowances 752,926 962,658
Inventories - 162,091
Prepaid expenses and other current assets 410,408 1,226,507
Short-term investments 1,880,000 -
Total current assets $ 3,581,363 $ 3,841,949
Goodwill 198,169 1,180,951
Intangible Assets - 113,321
Property, plant and equipment, net 953,936 7,074,437
Investments in affiliates 773,111 609,148
Investments-others 5,238,003 5,175,392
Deferred Income taxes - 356,684
Other non-current assets 539,720 507,300
Total long-term assets $ 7,702,939 $ 15,017,233
Total assets $ 11,284,302 $ 18,859,182
LIABILITIES AND STOCKHOLDERS' EQUITY
Current liabilities:
Short -term borrowings - 27,762
Trade payables 416,532 330,631
Accrued expenses 181,465 300,111
Loans - others - 189,680
Notes payable - 1,800,000
Other current liabilities 691,714 550,877
Total current liabilities $ 1,289,711 $ 3,199,061
Long -term borrowings 452,080 801,467
Loans - others 392,226 -
Notes payable 1,800,000 -
Other non-current liabilities - 910,583
Total long-term liabilities $ 2,644,306 $ 1,712,050
Total liabilities $ 3,934,017 $ 4,911,111
Stockholders' equity:
Common stock — $.0001 par value; 150,000,000 shares authorized; 23,265,531
issued and outstanding as of March 31, 2016 and 28,272,667 issued and
outstanding as of March 31, 2017. $ 2,827 $ 2,327
Additional paid-in capital 61,413,533 65,885,243
Accumulated other comprehensive income (2,047,780 ) (2,269,357 )
Retained earnings (Deficit) (52,009,459 ) (50,142,199 )
Total equity attributable to Parent $ 7,359,121 $ 13,476,014
Non-controlling interest $ (8,836 ) $ 472,057
Total stockholders' equity $ 7,350,285 $ 13,948,071
Total liabilities and stockholders' equity $ 11,284,302 $ 18,859,182
These financial statements should be read in connection with the accompanying
notes on Form 10-K for fiscal 2017
filed with the SEC on July 13, 2017.
INDIA GLOBALIZATION CAPITAL, INC. AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF OPERATIONS
(Audited)
(All amounts in USD, except number of shares and per share amounts)
Year ended March 31,
2017 2016
Revenues $ 580,372 $ 6,366,550
Cost of revenues (excluding depreciation) (362,135 ) (5,523,256 )
Selling, general and administrative expenses (1,875,344 ) (2,702,753 )
Depreciation (396,346 ) (728,741 )
Loss on investments / associates /joint ventures (932 ) (317,510 )
Operating income (loss) $ (2,054,385 ) $ (2,905,710 )
Interest expense (223,464 ) (213,928 )
Interest income 1,744 2,085
Profit on investments/associates and Joint Ventures 317,742 -
Other income, net 119,933 284,186
Income before income taxes and minority interest attributable to non-controlling
interest $ (1,838,430 ) $ (2,833,367 )
Income taxes benefit/ (expense) (14,431 ) (579 )
Net income/(loss) $ (1,852,861 ) $ (2,833,946 )
Non-controlling interests in earnings of subsidiaries 14,399 (25,702 )
Net income / (loss) attributable to common stockholders $ (1,867,260 ) $
(2,808,244 )
Earnings/(loss) per share attributable to common stockholders:
Basic $ (0.07 ) $ (0.17 )
Diluted $ (0.07 ) $ (0.17 )
Weighted-average number of shares used in computing earnings per share amounts:
Basic 25,658,544 16,387,290
Diluted 25,658,544 16,387,290
These financial statements should be read in connection with the accompanying
notes on Form 10-K for fiscal 2017
filed with the SEC on July 13, 2017.
About IGC
India Globalization Capital is engaged in the development of cannabis-based
combination therapies to treat Alzheimer’s, pain, nausea, eating disorders,
several end points of Parkinson’s, and epilepsy in humans, dogs and cats. In
support of this effort, IGC has assembled a portfolio of patent filings and four
lead product candidates addressing these conditions. The company is based in
Bethesda, Maryland.
For more information visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see forward-looking statements as discussed in detail in IGC's Form 10-K
for fiscal year ended March 31, 2017, and in other reports filed with the U.S.
Securities and Exchange Commission.
Contact Info:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
Pharmaceutical Leaders Pave the Way
to New Alzheimer’s Treatments
New York, NY – June 15, 2017 –
NetworkNewsWire.com News Coverage:
Alzheimer’s disease is a devastating brain disorder that now affects about 5.3
million Americans, accounting for one in nine people over the age of 65,
according to the Alzheimer’s Association. One in three seniors die with the
disease or another form of dementia, and, while treatments
 to
slow or stop the disease’s progression have been lacking, several pharmaceutical
companies are working to change that
India Globalization Capital, Inc. (NYSE: IGC) , which has products that
are backed by data of cannabis-based combination therapies to treat Alzheimer’s,
pain, seizures and a host of neurological and oncological issues, is lined up
with Biogen, Inc. (NASDAQ: BIIB), Anavex Life Sciences Corp. (NASDAQ: AVXL),
Axovant Sciences Ltd. (NASDAQ: AXON) and AC Immune SA (NASDAQ: ACIU) to develop
medications they hope will yield promising results in treating a difficult and
hard to treat illness. to
slow or stop the disease’s progression have been lacking, several pharmaceutical
companies are working to change that
India Globalization Capital, Inc. (NYSE: IGC) , which has products that
are backed by data of cannabis-based combination therapies to treat Alzheimer’s,
pain, seizures and a host of neurological and oncological issues, is lined up
with Biogen, Inc. (NASDAQ: BIIB), Anavex Life Sciences Corp. (NASDAQ: AVXL),
Axovant Sciences Ltd. (NASDAQ: AXON) and AC Immune SA (NASDAQ: ACIU) to develop
medications they hope will yield promising results in treating a difficult and
hard to treat illness.
The cost to the U.S economy from Alzheimer’s disease is estimated at $236
billion, including the costs of caring for individuals with the illness. Without
effective treatments, the economic impact will continue to climb.
While industry behemoths like Pfizer and Novartis are obvious contenders, IIndia
Globalization Capital, Inc. (NYSE: IGC)is the first cannabis
pharmaceutical company to focus on Alzheimer’s disease. While there are more
than a dozen publicly traded cannabis pharmaceutical stocks on the market, most
of them with market caps much larger than IGC’s $12 million, IGC is so far the
only one to apply its cannabis-therapies to the treatment of Alzheimer’s.
IGC is aggressively advancing its unique position, and earlier this week
announced ( http://nnw.fm/3QaWV ) that it has
acquired the exclusive rights to a THC-based treatment for Alzheimer’s, moving
the company one step closer to clinical tri als
for its Alzheimer’s product. als
for its Alzheimer’s product.
Under a definitive license agreement with the University of South Florida, IGC
is the exclusive licensee of a patent filing for the use of tetrahydrocannabinol
(THC) as a potential therapeutic agent for the disease. The patent, entitled
“THC as a Potential Therapeutic Agent for Alzheimer’s Disease,” claims discovery
of a new pathway in which low doses of THC bind to amyloid beta plaques and
prevent those plaques from aggregating on neurons, which is what occurs in
Alzheimer’s disease and causes cognitive decline.
Here’s why the application of TCH (tetrahydrocannabinol), which is the
psychoactive compound found in cannabis, is so exciting for the future of
Alzheimer’s treatment. THC has several known molecular pathways by which it
interacts with the body. Numerous studies have shown that low doses of THC can
slow the production of neural plaques (considered a key contributor to the
progression of Alzheimer’s), as well as decrease inflammation, prevent cell
death and stimulate cell growth.
The acquisition of IGC’s new patent filing provides another layer of protection
for the company’s proprietary formulation of IGC-AD1, which includes low-doses
of THC and is intended to disrupt the buildup of amyloid beta plaques and
relieve some of the worst symptoms of Alzheimer’s.
If the patent is granted, and it works, IGC will become the owner of a major
therapeutic pathway for THC interaction with the body, with significant
potential in treating this condition.
“Securing this licensing agreement from the University of South Florida
represents a major turning point for IGC as we now look to prepare several of
our key products for clinical trials,” IGC CEO Ram Mukunda stated in the press
release. “We have worked hard to assemble a strong development team and a
primary pipeline of four major products addressing large markets and possible
blockbuster indications utilizing cannabis-based therapies. We are putting the
finishing touches on our products, which may include filing additional patents,
and we very much expect to start pursuing clinical trials for our Alzheimer’s
product and others this year.”
IGC’s drug development pipeline also includes cannabinoid therapy that targets
human and veterinary epilepsy (
http://nnw.fm/eW8gb ). The company is developing numerous cannabis-based
therapies, with a potential international market reach.
There are some other companies working on Alzheimer's with very large
valuations, with
IGC bringing the potential benefits of cannabis to bear on the disease.
Biogen (NASDAQ: BIIB) has developed and tested aducanumab, a drug that helps
eliminate beta amyloid, a sticky plaque found in the brain of Alzheimer’s
patients. The antibody binds to the plaque, which has the potential to slow the
progression of symptoms such as memory loss, confusion, trouble finding words,
decreases in judgement, and changes in mood and personality. Phase 1 clinical
trials included 165 patients, concluding that high doses of the drug decreased
the level of amyloid plaque in the brain, as viewed using positron emission
tomography. Scientists noted the slowing of the progression of memory loss in
these patients as well.
Another company focused on the development of innovative therapies for
Alzheimer’s, other central nervous system diseases (CNS) and various types of
cancer, is New York-based Anavex Life Sciences (NASDAQ: AVXL). The company’s
lead product candidate, ANAVEX™ 2-73, is an oral drug currently in phase 2a
clinical trials for Alzheimer’s, and is also being tested to Parkinson’s and
other CNS diseases. Phase 1 trials for ANAVEX™ 2-73 were completed with
encouraging results attesting to the compound’s potential to halt or even
reverse the course of Alzheimer’s, as well as with an indication of other
anticonvulsant, neuroprotective and antidepressant properties. A phase 2
placebo-controlled trial is also underway to determine ANAVEX™2-73 effects on
Rett Syndrome, while a separate trial is being conducted for Parkinson’s
disease. In addition to ANAVEX™2-73, the company is developing several other
products targeting various symptoms and effects of Alzheimer’s.
Clinical studies are also underway for Axovant Sciences’ (NASDAQ: AXON) lead
product candidate, Intepirdine (RVT-101), and its effects on Alzheimer’s,
dementia and Lewy Body Dementia. The Mindset study for Alzheimer’s is a phase 3
international, double-blind, multi-center, placebo-controlled clinical trial
that aims to assess the tolerability, safety and efficacy of the drug for mild
and moderate cases of Alzheimer’s. If the trials are successful, the therapy
will be approved for commercialization across Europe and the United States.
Phase 2 studies are being conducted to evaluate the product’s performance for
dementia. A novel glycopyrrolate and cholinesterase combination is also being
developed for Alzheimer’s and Lewy Body Dementia and is currently in phase 1
trials.
One of the world’s largest Alzheimer’s treatment pipelines is being developed by
Swiss-based AC Immune SA (NASDAQ: ACIU). The company is working on seven
therapeutic and three diagnostic products in various stages of clinical trials,
with the potential to significantly improve treatment and even prevent
Alzheimer’s and other neurodegenerative diseases. All of AC Immune’s therapies
were discovered and developed via the company’s proprietary Morphomer™ and
SupraAntigen™ technology platforms. The lead product candidate, an anti-Abeta
antibody called Crenezumab with treatment and prevention potential, is currently
undergoing phase 3 clinical trials, in collaboration with Roche Holding AG (OTC:
RHHBY) subsidiary, Genentech.
The focus on Alzheimer’s of so many biopharmaceutical companies is
understandable, given the prevalence of this condition. In May 2017, Congress
signed into law a $400 million increase for Alzheimer’s research funding at the
National Institutes of Health (NIH) in the fiscal year 2017 budget, marking the
second consecutive year that Congress has approved a “historic” funding increase
for the disease.
This move demonstrates the urgency for the more than 5 million Americans living
with the disease – a number that’s forecast to increase to 16 million by 2050 –
and highlights the value of the lineup of companies working to advance their
efforts for a cure.
For more information on India Globalization Capital visit
India Globalization Capital (IGC)
About NetworkNewsWire
NetworkNewsWire (NNW) is an information service that provides to users (1)
access to our news aggregation and syndication servers, (2) enhanced press
release services, and (3) a full array of social communication solutions. As a
multifaceted financial news and content distribution company with an extensive
team of contributing journalists and writers, NNW is uniquely positioned to best
serve private and public companies that desire to reach a wide audience of
investors, consumers, journalists and the general public. NNW has an
ever-growing distribution network of more than 5,000 key syndication outlets
across the country. By cutting through the overload of information in today's
market, NNW brings its clients unparalleled visibility, recognition and brand
awareness. NNW is where news, content and information converge.
NetworkNewsWire (NNW)
New York, New York
www.NetworkNewsWire.com
212.418.1217 Office
Editor@NetworkNewsWire.com
Please see full terms of use and disclaimers on the NetworkNewsWire website
applicable to all content provided by NNW, wherever published or re-published:
http://NNW.fm/Disclaimer
DISCLAIMER: NetworkNewsWire (NNW) is the source of the Article and content set
forth above. References to any issuer other than the profiled issuer are
intended solely to identify industry participants and do not constitute an
endorsement of any issuer and do not constitute a comparison to the profiled
issuer. FN Media Group (FNM) is a third-party publisher and news dissemination
service provider, which disseminates electronic information through multiple
online media channels. FNM is NOT affiliated with NNW or any company mentioned
herein. The commentary, views and opinions expressed in this release by NNW are
solely those of NNW and are not shared by and do not reflect in any manner the
views or opinions of FNM. Readers of this Article and content agree that they
cannot and will not seek to hold liable NNW and FNM for any investment decisions
by their readers or subscribers. NNW and FNM and their respective affiliated
companies are a news dissemination and financial marketing solutions provider
and are NOT registered broker-dealers/analysts/investment advisers, hold no
investment licenses and may NOT sell, offer to sell or offer to buy any
security.
The Article and content related to the profiled company represent the personal
and subjective views of the Author, and are subject to change at any time
without notice. The information provided in the Article and the content has been
obtained from sources which the Author believes to be reliable. However, the
Author has not independently verified or otherwise investigated all such
information. None of the Author, NNW, FNM, or any of their respective
affiliates, guarantee the accuracy or completeness of any such information. This
Article and content are not, and should not be regarded as investment advice or
as a recommendation regarding any particular security or course of action;
readers are strongly urged to speak with their own investment advisor and review
all of the profiled issuer's filings made with the Securities and Exchange
Commission before making any investment decisions and should understand the
risks associated with an investment in the profiled issuer's securities,
including, but not limited to, the complete loss of your investment.
NNW & FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW and FNM undertake no obligation to update
such statements.
Media Contact e-mail: FN Media Group, LLC - editor@financialnewsmedia.com
(954)345-0611
News Source: NetworkNewsWire
IGC Acquires Exclusive Rights
to THC-based treatment for Alzheimer’s Disease
BETHESDA, Md., June 12, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
is pleased to announce that it has entered into a definitive license agreement
with the University of South Florida making IGC the exclusive licensee of the
U.S. patent filing entitled “THC as a Potential Therapeutic Agent for
Alzheimer’s Disease.” By acquiring this patent filing, IGC is protecting a
potential cannabis-based blockbuster treatment for America’s most expensive
disease.
Alzheimer’s Disease Market OverviewIn 2017 Medicare and Medicaid alone are
expected to spend $175 billion on patients diagnosed with Alzheimer’s. There are
currently over 5.3 million Americans with Alzheimer’s (AD). The cost of
Alzheimer’s has increased significantly and is expected to continue to surge
higher. The number of patients is expected to double over the next 20 years and
the direct costs are expected to exceed $450 billion per year in the next 12
years. There is still no accepted cure for Alzheimer’s Disease.
accepted cure for Alzheimer’s Disease.
The PatentTHC has several known molecular pathways by which it interacts with
the human body, including binding to the CB1 receptor, anti-oxidative effects,
and others. The patent filing claims discovery of a new pathway: low doses of
THC bind to amyloid beta plaques and prevent those plaques from aggregating on
neurons, which is what occurs in Alzheimer’s disease and causes cognitive
decline. If the patent is granted and proven, IGC will own a significant
therapeutic pathway by which THC interacts with the human body. The pathway has
incredible potential in treating Alzheimer’s disease and is now a burgeoning
research area. Acquiring this patent further supports IGC in protecting its
proprietary formulation IGC-AD1, which includes low-doses of THC and is intended
to disrupt the buildup of amyloid beta plaques and alleviate some of the worst
symptoms of Alzheimer’s disease.
Next Steps“Securing this licensing agreement from the University of South
Florida represents a major turning point for IGC as we now look to prepare
several of our key products for clinical trials,” explained Ram Mukunda, CEO of
IGC. “We have worked hard to assemble a strong development team and a primary
pipeline of four major products addressing large markets and possible
blockbuster indications utilizing cannabis-based therapies. We are putting the
finishing touches on our products, which may include filing additional patents,
and we very much expect to start pursuing clinical trials for our Alzheimer’s
product and others this year.” IGC also expects in the near future to share some
data that supports its formulation, the patent application, and the transition
to clinical trials as well as details of commercialization initiatives.
About IGC
India Globalization Capital Inc. is engaged in the development of cannabis-based
therapies to treat cachexia, nausea, vomiting, Parkinson’s Disease, Alzheimer’s
Disease, epilepsy, PTSD and other life altering conditions in humans and
animals. In support of this mission, IGC has assembled a portfolio of patent
filings for its phytocannabinoid-based treatments. The company is based in
Bethesda, Maryland.
For more information, visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements, Statistics, and Risk Factors
Please see cautionary statements related to Safe Harbor, forward-looking
statements, and risk factors discussed in India Globalization Capital, Inc.’s
Form 10-K for fiscal year ended March 31, 2016, and in subsequent reports,
including the June 12, 2017 report on Form 8-K, filed with the U.S. Securities
and Exchange Commission. Statistics on Alzheimer’s Disease are cited from the
website of the Alzheimer’s Association (alz.org).
Contact Info:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
Cannabis-Based Therapies with
Potential to Dull the Pain of Opioid Abuse
New York, NY – May 17, 2017 –
NetworkNewsWire.com News Coverage:
America is in an opioid epidemic. On an average day, 91 Americans will die from
an opioid-related overdose. That’s according to the CDC, which also reports that
the
 number
of prescription opioids sold in the U.S. has nearly quadrupled since 1999
despite no overall change in the amount of pain reported. The result is more
than half a million deaths by opioid overdose from 2000 to 2015. These sobering
figures reveal the desperate need for opioid substitutes, and cannabis-based
therapies for pain treatment are emerging as viable alternatives. The potential
rewards are significant for companies that can develop these therapies, and
among those growing in recognition are India Globalization Capital, Inc.
(NYSE: IGC), Vitality Biopharma, Inc. (OTC: VBIO), Cara Therapeutics,
Inc. (NASDAQ: CARA), Zynerba Pharmaceuticals, Inc. (NASDAQ: ZYNE) and AXIM
Biotechnologies, Inc. (OTC: AXIM). number
of prescription opioids sold in the U.S. has nearly quadrupled since 1999
despite no overall change in the amount of pain reported. The result is more
than half a million deaths by opioid overdose from 2000 to 2015. These sobering
figures reveal the desperate need for opioid substitutes, and cannabis-based
therapies for pain treatment are emerging as viable alternatives. The potential
rewards are significant for companies that can develop these therapies, and
among those growing in recognition are India Globalization Capital, Inc.
(NYSE: IGC), Vitality Biopharma, Inc. (OTC: VBIO), Cara Therapeutics,
Inc. (NASDAQ: CARA), Zynerba Pharmaceuticals, Inc. (NASDAQ: ZYNE) and AXIM
Biotechnologies, Inc. (OTC: AXIM).
According to a 2016 report published by Transparency Market Research, analysts
surmise that almost 20 percent of the world’s population currently suffers from
chronic pain(1). As such, the global pain management therapeutics market is
expected to exhibit a 3.7 percent CAGR through 2024, with market valuation
increasing to US $83.0 billion by the same year.
A closer look at chronic pain explains this growth. Terminal illness, for one,
is frequently accompanied by pain so agonizing, severe, and difficult to treat
that death can seem like a preferable option to the patient. A key disadvantage
of currently available drug therapies for chronic severe pain is that opioid-based
drugs can create various side effects, including hallucinations, sedation,
nausea, respiratory depression, constipation and dysphoria. Pain treatment also
poses a significant challenge to health care professionals because it frequently
debilitates patients in ways that hamper their day-to-day functionality, which,
by extension, poses a huge productivity problem among U.S. citizens. In 2012,
the annual cost of pain in the United States was estimated at between $560
billion and $635 billion—eclipsing the costs of national priority health
conditions.
In light of this information, imagine for a moment the inconceivable potential
of a cannabis-based combination therapy that could effectively address a large
market indication. An achievement of this sort would propel an undiscovered
stock straight into the spotlight. Such is the case and possibility with
India Globalization Capital, Inc.
(NYSE: IGC),.
IGC aims to formulate and commercialize cannabinoid compounds as an alternative
to addictive long-term opioid treatments, endeavoring to do so through a focus
on combination therapy. The company is developing a portfolio of
phytocannabinoid-based therapies to treat a broad range of therapeutic
indications, including neuropathic and cancer pain. IGC has applied for a U.S.
patent to treat pain based on a novel therapy that utilizes cannabinoid
extracts. This therapy employs a cream that is applied through the use of
various delivery technologies and has indications in both humans and other
mammals, including dogs and cats. The company plans to conduct metabolic
profiling and to run trials in the near term. IGC’s patent filing (IGC-501) is a
cannabis-based formulation that addresses neuropathic and arthritic pain via
various delivery techniques.
IGC’s innovative and exciting strategy is to leverage its “first mover” position
to become a leader within the phytocannabinoid-based combination therapy
specialty pharmaceutical sector. Developing cannabis-based combination therapies
represents a huge and unique opportunity in this emerging specialty
pharmaceutical sector. Obtaining FDA approval for combination therapy is
typically significantly quicker and less costly than new drug applications,
which means IGC can potentially bring its cannabis-based pharmaceutical products
to market in a timely and thus cost-effective manner. With a market cap of
approximately $11.0 million, a fraction of that of its peer group, IGC is poised
to explode once its cannabis-based pain therapies obtain approval and can be
brought to the market.
Another company taking aim at bringing cannabis-based therapies to market is
Vitality Biopharma (OTCQB: VBIO), which has a market cap of approximately $51.7
million. A cure development company, Vitality Biopharma is focused on utilizing
cannabinoid “prodrugs” to treat serious neurological and inflammatory disorders
and to provide targeted therapies with the potential to offer long-term or
durable relief and possibly help patients recover lost function.
Prodrugs are compounds or medications that, upon administration, are converted
inside the body into a pharmacologically active drug that may already have a
lengthy history of clinical investigation and use. As reference drugs may
already have independent verification of their safety and effectiveness, some
prodrugs can potentially be quickly approved by demonstrating comparable bioavailability or bioequivalence. A prodrug can
simultaneously be much more marketable because of its ability to eradicate
undesirable side effects or commercial aspects.
demonstrating comparable bioavailability or bioequivalence. A prodrug can
simultaneously be much more marketable because of its ability to eradicate
undesirable side effects or commercial aspects.
As public interest in medical marijuana applications continues to increase,
clinical trials sponsored by academic investigators for using cannabinoids in
the treatment of profitable, large-market disease indications is also
increasing. Vitality Biopharma’s ability to quickly create proprietary prodrug
versions of CBD, THC, CBVD and others positions the company to benefit from
emerging data and increasing medical community recognition of the potential pain
relief and anti-inflammatory effects of cannabinoids. It has become a
well-established fact that cannabis has the potential to provide relief of pain
and inflammation in certain disease indications, and Vitality Biopharma’s
prodrugs have the potential to exert the same positive therapeutic effects as
current therapies, with noteworthy improvements. Vitality Biopharma has filed
intellectual property applications and is seeking global patent protection
through 2035 with strong composition matter claims for prodrugs of THC, CBD and
CBDV, as well as for the company’s proprietary prodrug biosynthesis platform
that utilizes enzymatic glycosylation.
Another company applying the medical properties of cannabis for the treatment of
pain is Cara Therapeutics (NASDAQ: CARA), with a market cap of approximately
$539.6 million. Cara Therapeutics is a clinical-stage biotechnology company
focused on developing and commercializing new chemical entities, which are
designed to lessen pain and pruritus by selectively targeting peripheral kappa
opioid receptors. The company is developing a novel and proprietary class of
product candidates designed to target the peripheral nervous system of the body
and that have shown initial efficacy in patients suffering with
moderate-to-severe pain. This has been demonstrated without creating many of the
unwanted side effects that are normally associated with current pain
therapeutics.
Studies conducted to assess the effects of cannabis have led to a recent
discovery of an endogenous system of ligands within humans that are involved in
various physiological processes, including pain and inflammation. The primary
naturally occurring ligands for this system activate numerous cannabinoid
receptors, including CB1 and CB2 receptors. CB1 receptors and associated ligands
are primarily localized in the brain, while CB2 receptors are chiefly found in
peripheral tissues—in particular immune cells like leukocytes and mast cells,
which have been shown to have involvement in pain and inflammatory responses.
Cara Therapeutics is developing lead molecules that selectively modulate
peripheral CB receptors without targeting CNS cannabinoid receptors. The company
will initially develop peripheral CB receptor modulators as a novel therapeutic
approach for neuropathic pain. CR701, the company’s most advanced CB compound,
is currently in preclinical development.
Zynerba Pharmaceuticals (NASDAQ: ZYNE), with a market cap of approximately
$255.8 million, is also seeking to treat a variety of severe health conditions,
including peripheral neuropathic pain—in this case by applying synthetic
cannabinoid therapeutics that are formulated for transdermal delivery. A
clinical-stage pharmaceutical company, Zynerba Pharmaceuticals currently has two
lead product candidates in its development pipeline: ZYN002 and ZYN001. These
product candidates are being evaluated in five therapeutic indications. ZYN002
is the first and, currently, the only synthetic CBD, which is a non-psychoactive
cannabinoid. It has been formulated as a patent-protected permeation-enhanced
gel for transdermal delivery through the skin into the body’s circulatory system
and is in phase 2 clinical development. ZYN001 is a prodrug of THC that
facilitates transdermal delivery through the skin and into the circulatory
system through a patch. A phase 1 clinical trial of ZYN001 is planned to
commence in 2017.
AXIM Biotechnologies (OTCQB: AXIM), with a market cap of approximately $410.5
million, is focused on the research, development and production of cannabinoid-based
pharmaceutical products and supplements. The company owns the patent for the use
of chewing gum as a cannabinoid delivery method, and its MedChew RX gum will
soon commence phase 3 clinical trials for the treatment of pain and spasticity
related to multiple sclerosis. This pharmaceutical, functional chewing gum
contains equal portions of THC and CBD. Thanks to the company’s proprietary
technology, various problems related to the water insolubility of cannabinoids
have been addressed and resolved. Based on AXIM’s proprietary intellectual
property, a new direct and controlled slow-release nano-technology delivery
method is planned to be investigated. Various other R&D projects are also being
considered that involve AXIM’s proprietary intellectual property.
There is no denying the power and potential of cannabis-based therapies for pain
management, and investors have the opportunity to scout the playing field of
this market to identify the most promising companies. This growing segment of
the health care field increasingly demonstrates favorability by addressing key
health issues that affect people in the United States and around the world.
Sources:
(1) Transparency Market Research
http://nnw.fm/uL54B
For more information on India Globalization Capital visit
India Globalization Capital (IGC)
About NetworkNewsWire
NetworkNewsWire (NNW) is an information service that provides to users (1)
access to our news aggregation and syndication servers, (2) enhanced press
release services, and (3) a full array of social communication solutions. As a
multifaceted financial news and content distribution company with an extensive
team of contributing journalists and writers, NNW is uniquely positioned to best
serve private and public companies that desire to reach a wide audience of
investors, consumers, journalists and the general public. NNW has an
ever-growing distribution network of more than 5,000 key syndication outlets
across the country. By cutting through the overload of information in today's
market, NNW brings its clients unparalleled visibility, recognition and brand
awareness. NNW is where news, content and information converge.
NetworkNewsWire (NNW)
New York, NY
www.NetworkNewsWire.com
212.418.1217 Office
Editor@NetworkNewsWire.com
Please see full terms of use and disclaimers on the NetworkNewsWire website
applicable to all content provided by NNW, wherever published or re-published:
http://NNW.fm/Disclaimer
DISCLAIMER: NetworkNewsWire (NNW) is the source of the Article and content set
forth above. References to any issuer other than the profiled issuer are
intended solely to identify industry participants and do not constitute an
endorsement of any issuer and do not constitute a comparison to the profiled
issuer. FN Media Group (FNM) is a third-party publisher and news dissemination
service provider, which disseminates electronic information through multiple
online media channels. FNM is NOT affiliated with NNW or any company mentioned
herein. The commentary, views and opinions expressed in this release by NNW are
solely those of NNW and are not shared by and do not reflect in any manner the
views or opinions of FNM. Readers of this Article and content agree that they
cannot and will not seek to hold liable NNW and FNM for any investment decisions
by their readers or subscribers. NNW and FNM and their respective affiliated
companies are a news dissemination and financial marketing solutions provider
and are NOT registered broker-dealers/analysts/investment advisers, hold no
investment licenses and may NOT sell, offer to sell or offer to buy any
security.
The Article and content related to the profiled company represent the personal
and subjective views of the Author, and are subject to change at any time
without notice. The information provided in the Article and the content has been
obtained from sources which the Author believes to be reliable. However, the
Author has not independently verified or otherwise investigated all such
information. None of the Author, NNW, FNM, or any of their respective
affiliates, guarantee the accuracy or completeness of any such information. This
Article and content are not, and should not be regarded as investment advice or
as a recommendation regarding any particular security or course of action;
readers are strongly urged to speak with their own investment advisor and review
all of the profiled issuer's filings made with the Securities and Exchange
Commission before making any investment decisions and should understand the
risks associated with an investment in the profiled issuer's securities,
including, but not limited to, the complete loss of your investment.
NNW & FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW and FNM undertake no obligation to update
such statements.
Media Contact e-mail: FN Media Group, LLC - editor@financialnewsmedia.com
(954)345-0611
News Source: NetworkNewsWire
IGC Appoints Medical Advisor
Craig Cheifetz, M.D. to Consult on the Development of Cannabis-Based,
Combination Therapies
BETHESDA, Md., May 16, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
announces that Craig Cheifetz, M.D. has joined the company as an advisor to
provide guidance on clinical trials, biotechnology, neuroscience, immunology and
microbiology.
Dr. Cheifetz is currently Regional Dean at Virginia Commonwealth University
Inova Fairfax Campus and is the Medical Director of Inova VIP 360 (Northern
Virginia's premier Concierge Medicine Program). Dr. Cheifetz received his M.D.
from th e
State University of New York College at Buffalo and trained in Internal Medicine
at Georgetown University. From 2011 to 2013, he served as the National GRMC
Chairman. e
State University of New York College at Buffalo and trained in Internal Medicine
at Georgetown University. From 2011 to 2013, he served as the National GRMC
Chairman.
“I welcome Dr. Cheifetz to the IGC advisory team and look forward to his
contributions as we move forward in developing cannabis-based, combination
therapies. We remain committed to accelerating our initiatives and building a
robust portfolio of compounds to address large market conditions,” states Ram
Mukunda, CEO of IGC.
About IGC
India Globalization Capital is engaged in the development of cannabis-based
therapies to treat pain, PTSD, seizures, cachexia, chronic and terminal
neurological and oncological diagnoses, and other life altering conditions. In
support of this mission, IGC has assembled a portfolio of patent filings for its
phytocannabinoid-based treatments. The company is based in Bethesda, Maryland.
For more information visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see forward-looking statements as discussed in detail in IGC's Form 10-K
for fiscal year ended March 31, 2016, and in subsequent reports filed with the
U.S. Securities and Exchange Commission.
Contact Info:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
Paws Firmly Planted for Growth in
Multibillion-Dollar Pet Health Market
New York, NY – May 3, 2017 –
NetworkNewsWire.com News Coverage:
Pets are the loyal, much-loved companions we humans can’t seem to live without,
and when a beloved pet becomes ill, owners will generally do whatever it takes
to restore these beloved companion animals to health. Various public companies
are accomplishing the two-fold mission of
 improving
the health of our four-footed friends while giving investors the opportunity to
capitalize on a multibillion-dollar industry. India Globalization Capital,
Inc.
(NYSE: IGC), VCA, Inc. (NASDAQ: WOOF), PetMed Express, Inc. (NASDAQ:
PETS), Blue Buffalo Pet Products, Inc. (NASDAQ: BUFF) and IDEXX Laboratories,
Inc. (NASDAQ: IDXX) are just some of the companies blazing lucrative trails in
the pet health market and successfully tapping into its virtually limitless
opportunities. improving
the health of our four-footed friends while giving investors the opportunity to
capitalize on a multibillion-dollar industry. India Globalization Capital,
Inc.
(NYSE: IGC), VCA, Inc. (NASDAQ: WOOF), PetMed Express, Inc. (NASDAQ:
PETS), Blue Buffalo Pet Products, Inc. (NASDAQ: BUFF) and IDEXX Laboratories,
Inc. (NASDAQ: IDXX) are just some of the companies blazing lucrative trails in
the pet health market and successfully tapping into its virtually limitless
opportunities.
In 2016 alone, U.S. consumers spent over $65 billion on pet-related products
(1). According to a 2013 study published by the U.S. Bureau of Labor Statistics,
U.S. households own approximately 218 million pets, and pet-related expenses for
the average U.S. household amounts to about 1 percent of their total annual
spending. Further, the North American Pet Health Insurance Association reports
that pet health insurance premiums hit $774 million in the U.S. in 2015, with
1.6 million pets being insured by the end of that year. Clearly, consumers are
interested in the health and wellbeing of their pets, and they’re showing it
with dollars.
The veterinary market is a veritable cash cow - if readers will pardon the pun -
that offers virtually limitless investment potential.
One pioneering company, India Globalization Capital, Inc.
(NYSE: IGC) is forging new paths in the medical marijuana market by
exploring the potential health applications of cannabis in domesticated animals.
IGC is engaged in the development of phytocannabinoid-based treatments to
address pain and a variety of medical conditions, including marijuana-based
therapies to treat seizures in dogs and cats.
marijuana-based
therapies to treat seizures in dogs and cats.
Treatment for pet seizures represents a surprisingly large market. Statistics
indicate that between 1 percent and more than 5 percent of dogs have some sort
of seizure disorder, and certain canine breeds with hereditary epilepsy may have
as high as 15 to 20 percent incidence of seizures. Abnormal brain activity is
frequently the culprit behind canine and feline seizures, and these seizures can
result in both subtle and violent convulsions, either of which is most alarming
for a pet owner to witness.
IGC offers a novel therapy using cannabinoid extracts to treat such seizures in
pets. The company has applied for a U.S. patent based on this therapy, which can
be administered via various delivery technologies and has indications for
mammals, including dogs and cats. This therapy has the potential to be useful in
humans, as well. IGC anticipates conducting metabolic profiling and commencing
trials.
Another company aimed at capitalizing on the opportunities of the pet health
market is VCA (NASDAQ: WOOF). This veterinary services company has become one of
the largest families of animal care providers in the United States, operating
more than 800 veterinary hospitals across the U.S. and in five Canadian
provinces. The company also provides diagnostic services and supplies high-tech
imaging to over 17,000 independent veterinary hospitals. Through its Camp Bow
Wow subsidiary, VCA additionally provides doggy daycare and boarding services at
more than 130 franchised locations throughout the United States.
Online pet pharmaceutical company PetMed Express (NASDAQ; PETS), also known
under the name 1-800-PetMeds, is the largest pet pharmacy in America, having
served over 9 million customers and counting. PetMeds is a licensed pharmacy
that dispenses FDA/EPA-approved medications for animals—the same products
available from veterinarians—and employs the largest number of veterinary
pharmacists in the world.
Blue Buffalo Pet Products (NASDAQ: BUFF) approaches pet health in another way,
offering natural, healthy pet foods to help prevent—and in some case treat—pet
illness and to foster the health of America’s pets in a nutrition-based way. The
company currently has approximately 6 percent share of the general pet food
industry, feeding 164 million pets in the U.S. and counting. The company’s
products include veterinarian- and nutritionist-formulated therapeutic pet foods
that can only be purchased through veterinary offices.
Pet health care innovator IDEXX Laboratories (NASDAQ: IDXX) serves veterinarians
across the globe with a wide array of diagnostic and information
technology-based services and products. These products better equip and enable
veterinarians to offer advanced medical care, bolster staff efficiency, and
build practices that are more economically successful. The company is also a
leading global provider of diagnostic tests and information for livestock and
poultry, as well as tests that gauge the quality and safety of water and milk.
Among its products, IDEXX offers the very first low-dose radiography system in
veterinary medicine, as well as a test that enables veterinarians to detect
acute kidney injury and chronic kidney disease in pets earlier than ever before.
IDEXX Laboratories products are sold in over 175 countries.
It’s no secret that consumers love their pets, and a multibillion-dollar global
industry is positive proof. Companies, such as those named, that are in the
business of serving the health interests of pets provide a virtually limitless
wellspring of investment opportunities in a market that is ever-growing and
boasts impressive longevity to rival any other market segment in existence.
For more information on India Globalization Capital please visit India
Globalization Capital, Inc.
(NYSE: IGC)
Editorial Sources:
(1) American Pet Products Association:
http://nnw.fm/V66et
About NetworkNewsWire
NetworkNewsWire (NNW) is an information service that provides to users (1)
access to our news aggregation and syndication servers, (2) enhanced press
release services, and (3) a full array of social communication solutions. As a
multifaceted financial news and content distribution company with an extensive
team of contributing journalists and writers, NNW is uniquely positioned to best
serve private and public companies that desire to reach a wide audience of
investors, consumers, journalists and the general public. NNW has an
ever-growing distribution network of more than 5,000 key syndication outlets
across the country. By cutting through the overload of information in today's
market, NNW brings its clients unparalleled visibility, recognition and brand
awareness. NNW is where news, content and information converge.
http://www.NetworkNewsWire.com
Please see full terms of use and disclaimers on the NetworkNewsWire website
applicable to all content provided by NNW, wherever published or re-published:
http://NNW.fm/Disclaimer
DISCLAIMER: NetworkNewsWire (NNW) is the source of the Article and content set
forth above. References to any issuer other than the profiled issuer are
intended solely to identify industry participants and do not constitute an
endorsement of any issuer and do not constitute a comparison to the profiled
issuer. FN Media Group (FNM) is a third-party publisher and news dissemination
service provider, which disseminates electronic information through multiple
online media channels. FNM is NOT affiliated with NNW or any company mentioned
herein. The commentary, views and opinions expressed in this release by NNW are
solely those of NNW and are not shared by and do not reflect in any manner the
views or opinions of FNM. Readers of this Article and content agree that they
cannot and will not seek to hold liable NNW and FNM for any investment decisions
by their readers or subscribers. NNW and FNM and their respective affiliated
companies are a news dissemination and financial marketing solutions provider
and are NOT registered broker-dealers/analysts/investment advisers, hold no
investment licenses and may NOT sell, offer to sell or offer to buy any
security.
The Article and content related to the profiled company represent the personal
and subjective views of the Author, and are subject to change at any time
without notice. The information provided in the Article and the content has been
obtained from sources which the Author believes to be reliable. However, the
Author has not independently verified or otherwise investigated all such
information. None of the Author, NNW, FNM, or any of their respective
affiliates, guarantee the accuracy or completeness of any such information. This
Article and content are not, and should not be regarded as investment advice or
as a recommendation regarding any particular security or course of action;
readers are strongly urged to speak with their own investment advisor and review
all of the profiled issuer's filings made with the Securities and Exchange
Commission before making any investment decisions and should understand the
risks associated with an investment in the profiled issuer's securities,
including, but not limited to, the complete loss of your investment.
NNW & FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW and FNM undertake no obligation to update
such statements.
Media Contact e-mail: FN Media Group, LLC - editor@financialnewsmedia.com
(954)345-0611
News Source: NetworkNewsWire
India Globalization Capital,
Inc. (IGC) Engages NetworkNewsWire for Corporate Communications Solutions
BETHESDA, Md., April 25, 2017 -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
engaged in the development of cannabis-based combination therapies to treat a
variety of life altering conditions, announces that it has engaged the expertise
of NetworkNewsWire ("NNW"), a multifaceted financial news and publishing company
that delivers a new generation of social communication solutions, news
aggregation and syndication, and enhanced news release services.
"We have an exciting pipeline of phytocannabinoid-based treatments based on our
formulation for pain. As we move toward pre clinical and clinical trials, we are
working with NetworkNewsWire to make sure the investment community is aware of
the opportunities created by our progress -- both from a corporate and broader
medical standpoint," states Ram Mukunda, CEO of IGC.
aware of
the opportunities created by our progress -- both from a corporate and broader
medical standpoint," states Ram Mukunda, CEO of IGC.
NNW's strategies help public and private organizations find their voice and
build market visibility. As part of the Client-Partner relationship with IGC,
NNW will leverage its investor-based distribution network of over 5,000 key
syndication outlets, various newsletters, social media channels, blogs, and
other outreach tools to generate greater brand awareness for the Company.
"IGC occupies a unique position in the medical cannabis field by focusing on
combination therapies for a number of indications, including possible veterinary
applications," states Sherri Franklin, director of Content Marketing for NNW.
"It is an interesting story to dig into, and we look forward to helping IGC
prime its corporate message to effectively tell this story to existing and
potential shareholders."
About India Globalization Capital (IGC)
India Globalization Capital is engaged in the development of cannabis-based
therapies to treat pain, PTSD, seizures, cachexia, chronic and terminal
neurological and oncological diagnoses, and other life altering conditions. In
support of this mission, IGC has assembled a portfolio of patent filings for its
phytocannabinoid-based treatments. The company is based in Bethesda, Maryland.
For more information, visit http://www.igcinc.us
About NetworkNewsWire
NetworkNewsWire (NNW) provides news aggregation and syndication, enhanced press
release services and a full array of social communication solutions. As a
multifaceted financial news and distribution company with an extensive team of
 journalists
and contributing writers, NNW is uniquely positioned to best serve private and
public companies who need to reach a wide audience of investors, consumers,
journalists and the general public. NNW has an ever-growing distribution network
of more than 5,000 key syndication outlets across the country. By cutting
through the overload of information in today's market, NNW brings its clients
unparalleled visibility, recognition and brand awareness. NNW is where news,
content and information converge. journalists
and contributing writers, NNW is uniquely positioned to best serve private and
public companies who need to reach a wide audience of investors, consumers,
journalists and the general public. NNW has an ever-growing distribution network
of more than 5,000 key syndication outlets across the country. By cutting
through the overload of information in today's market, NNW brings its clients
unparalleled visibility, recognition and brand awareness. NNW is where news,
content and information converge.
For more information, visit https://www.NetworkNewsWire.com
Please see full disclaimers on the NetworkNewsWire website applicable to all
content provided by NNW, wherever published or re-published: http://nnw.fm/Disclaimer
Forward-Looking Statements
This release contains forward-looking statements within the meaning of Section
27A of the Securities Act of 1933, as amended and Section 21E of the Securities
Exchange Act of 1934, as amended. All forward-looking statements are inherently
uncertain as they are based on current expectations and assumptions concerning
future events or future performance of the company. Readers are cautioned not to
place undue reliance on these forward-looking statements, which are only
predictions and speak only as of the date hereof. In evaluating such statements,
prospective investors should review carefully various risks and uncertainties
identified in this release and matters set in the company's SEC filings. These
risks and uncertainties could cause the company's actual results to differ
materially from those indicated in the forward-looking statements.
NNW Contact:
NetworkNewsWire (NNW)
New York, New York
www.NetworkNewsWire.com
212.418.1217 Office
Email Contact
Source: NetworkNewsWire
NYSE MKT Extends India
Globalization Capital Compliance Plan Period
BETHESDA, Md., April 21, 2017 (GLOBE NEWSWIRE) -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
announces that on April 20, 2017 it received notice from NYSE MKT LLC (“the
Exchange”) that it has accepted the Company’s plan to regain compliance with the
Exchange’s continued listing standards.
The Exchange also confirmed that the Company has made a reasonable demonstration
of its ability to regain compliance with Section 704 of the Company Guide which
addresses holding an annual meeting of its stockholders. The Exchange granted
the Company an extension until October 16, 2017 to regain compliance with
Section 704. Failure to make progress consistent with the “Plan of Compliance”
or to regain compliance with the continued listing standards by the end of the
applicable extension period could result in the Company’s shares being delisted
from the Exchange.
granted
the Company an extension until October 16, 2017 to regain compliance with
Section 704. Failure to make progress consistent with the “Plan of Compliance”
or to regain compliance with the continued listing standards by the end of the
applicable extension period could result in the Company’s shares being delisted
from the Exchange.
The Company is diligent in its effort to ensure compliance with Exchange
requirements within the period granted, subject to periodic review by the
Exchange.
About IGCIndia Globalization Capital is engaged in the development of
cannabis-based therapies to treat pain, PTSD, seizures, cachexia, chronic and
terminal neurological and oncological diagnoses, and other life altering
conditions. In support of this mission, IGC has assembled a portfolio of patent
filings for its phytocannabinoid-based treatments. The company is based in
Bethesda, Maryland.
For more information visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking StatementsPlease see forward-looking statements as discussed in
detail in IGC's Form 10-K for fiscal year ended March 31, 2016, and in
subsequent reports filed with the U.S. Securities and Exchange Commission.
Contact Info:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
IGC Files International
Patents for IGC-501 Compound Indicated for Neuropathic Pain
BETHESDA, Md., April 10, 2017 (GLOBE NEWSWIRE) -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
announces today that it has filed patent applications for IGC-501 in Canada,
Israel, and Europe in support of its global cannabis-based combination therapy
development initiatives.
IGC-501 is indicated for neuropathic pain. The pain market represents a
significant component of the healthcare system and The Journal of Pain in
September 2012 reported that the annual estimated national cost of pain ranges
from $560 billion to $635 billion. In addition, abuse of prescription opioids is
estimated to cost about $25 billion.
The international landscape embraces advancements in medical cannabis with
countries like Germany, Israel and Canada being in the forefront. We aspire to
bring our cannabis-based compounds to these markets and position ourselves for
sustained growth. Canada’s recent introduction of legislation to legalize
cannabis and Germany’s initiatives on medical cannabis are particularly
encouraging on a global perspective. It should be noted that filing a patent
does not guarantee that a patent will be issued in the future.
“We continue to strengthen our IP position in cannabis-based combination
therapies and have progressed to include global patent filings. IGC is committed
to addressing high potential international markets as a first mover in this
emerging segment of cannabis-based combination therapy pharmaceuticals,”
concludes Ram Mukunda, CEO.
About IGC
India Globalization Capital is engaged in the development of cannabis-based
therapies to treat pain, PTSD, seizures, cachexia, chronic and terminal
neurological and oncological diagnoses, and other life altering conditions. In
support of this mission, IGC has assembled a portfolio of patent filings for its
phytocannabinoid-based treatments. The company is based in Bethesda, Maryland.
For more information visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see risk factors discussed in India Globalization Capital, Inc.’s Form
10-K for fiscal year ended March 31, 2016, and in subsequent reports filed with
the U.S. Securities and Exchange Commission.
Contact Info:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
IGC Sells Malaysian Hotel
Investment Interest
Consolidates Corporate Focus to the Development of Cannabis-Based Combination
Therapies
BETHESDA, Md., April 05, 2017 (GLOBE NEWSWIRE) -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
announces today that it has sold its ten percent holding in Brilliant Hallmark
for a consideration of four million shares of IGC.
As a result of this transaction, 4,000,000 IGC common shares previously issued
to Brilliant Hallmark, on August 4, 2016, will be returned and retired, thereby
reducing the outstanding IGC shares from approximately 28,272,667 million before
the sale to approximately 24,272,667 million after the sale. In addition, the
Brilliant Hallmark investment will be removed from the IGC balance sheet with an
associated reduction of approximately $1.88 million. The Company does not expect
to record a gain or loss from this transaction.
a gain or loss from this transaction.
“This strategic move is consistent with our Board’s mandate to consolidate our
corporate focus on developing cannabis-based therapies while efficiently
disposing with all non-core assets. In the past twelve months, the Company has
retired almost seven million common shares, and exited from non-core assets in
China, Hong Kong, and Malaysia while closing overseas subsidiaries and offices.
We remain committed to streamlining operations and this transaction represents
another significant step in executing on our initiatives,” states Ram Mukunda,
CEO of IGC.
About IGC
India Globalization Capital is engaged in the development of cannabis-based
therapies to treat pain, PTSD, seizures, cachexia, chronic and terminal
neurological and oncological diagnoses, and other life altering conditions. In
support of this mission, IGC has assembled a portfolio of patent filings for its
phytocannabinoid-based treatments. The company is based in Bethesda, Maryland.
For more information visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Please see risk factors discussed in India Globalization Capital, Inc.’s Form
10-K for fiscal year ended March 31, 2016, and in subsequent reports filed with
the U.S. Securities and Exchange Commission.
Contact Info:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
Pain-Reducing Combination
Therapies Proving Successful thanks to Innovative, Specialty Pharma Players
New York, NY – March 30, 2017 –
NetworkNewsWire.com News Coverage:
Combination therapies that use cannabis-based pain reduction methods without the
negative side effects of opioids are looking to be a positive growth opportunity
among specialty pharmaceuticals, particularly for postoperative and chronic pain
relief. Leading specialty pharma companies such as India Globalization
Capital, Inc. (NYSE:
IGC), Cara Therapeutics, Inc. (NASDAQ: CARA), Zynerba Pharmaceuticals,
Inc. (NASDAQ: ZYNE), and INSYS Therapeutics, Inc. (NASDAQ: INSY) are tirelessly
working to expand combination therapeutics product lines, while Pacira
Pharmaceuticals, Inc. (NASDAQ: PCRX) developing an opioid alternative drug
intended to reduce pain after surgery.

To date, it is estimated that the annual national cost of pain is between $560
billion and $635 billion – more than priority health conditions among the
nation. Sadly, the treatment of postoperative pain remains a daunting challenge
for health care professionals, leaving patients debilitated in ways that can
affect their daily functioning and, in turn, have a significant impact on our
nation’s overall productivity. At present, patients face a significant
disadvantage when using opioid drugs to treat postoperative and chronic severe
pain, and usage thereof can lead to impactful side effects such as nausea,
hallucinations, respiratory depression, constipation, and sedation.
While opioids have been widely accepted as a standard therapy to treat acute
post-operative pain, the frequent adverse effects and burden of the cost of care
has proven to be a barrier for patients who are unable to receive optimal dosing
or adhered to their prescribed treatment. The administration of two opioids is
not usually recommended as a treatment for moderate to severe acute pain;
however, cannabis-based combination therapies present a unique opportunity in
the health sector. Cannabis pharma company India Globalization Capital, Inc.
(NYSE:
IGC) has carved an impressive course in the combination therapy market
as a “first mover” to combine existing drugs with cannabis. IGC recently filed a
patent for a formulation that the company claims will reduce the side effects of
single drugs by combining them with a cannabinoid. In particular, IGC has filed
for a provisional patent for the treatment of several eating disorders that uses
a combination of compounds and cannabis extracts. The combo therapy is
ear-marked for both veterinary and human consumption.
After three years of building a sturdy IP portfolio, IGC is now set to obtain
funding to begin both pre-clinical and clinical trials for combination
therapies, for which it has started reviewing international medical facilities.
Fortunately, securing FDA approval for such therapies, which include the
treatment of seizures, neuropathic pain and eating disorders, tends to be
swifter and less costly than applying for new drug approval. It is the company’s
aim to leverage its unique first-mover position and bring its cannabis-based
pharmaceuticals to market in a cost-effective and prompt manner.
Meanwhile, Pacira Pharmaceuticals (NASDAQ: PCRX), which is collaborating with
Trinity Health to develop an alternative approach to opioids for pain
management, recently announced positive results for its medication for opioid
reduction in a phase 4 clinical trial. During the trial, a pain regimen along
with EXPAREL® was tested against a bupivacaine-based pain regimen that involved
other drugs in 139 patients who were undergoing knee replacements. EXPAREL,
currently used to produce postsurgical analgesia, combines bupivacaine with the DepoFoam® platform
to enable the delivery of bupivacaine over a desired period of time, providing
significant reductions in pain with up to a 45 percent decrease in opioid
consumption.
produce postsurgical analgesia, combines bupivacaine with the DepoFoam® platform
to enable the delivery of bupivacaine over a desired period of time, providing
significant reductions in pain with up to a 45 percent decrease in opioid
consumption.
In the phase 4 clinical trial, EXPAREL achieved statistical significance for its
co-primary endpoints for postsurgical pain and opioid reduction, as well as
achieved statistical significance for key secondary endpoints. In a press
release announcing the results, Pacira said it continues to analyze further
secondary endpoints.
Cara Therapeutics (NASDAQ: CARA) is also working to change pain management on a
fundamental level. Rather than concentrating on improving old compounds, the
company is developing a new class of medicine referred to as KORAs – Kappa
Opioid Receptor Agonists. This medication is aimed at targeting different
receptors in the body to treat pain in an unconventional, yet more effective,
manner. Up to now, Cara’s core product has shown favorable results in phase 2
clinical trials for pain relief and does not elicit the usual side effects
patients would normally experience when using opioids such as hydrocodone,
morphine, and non-steroidal anti-inflammatories.
Two more candidates for combination therapies come from Zynerba Pharmaceuticals
(NASDAQ: ZYNE). ZYN001 and ZYN002 are both being evaluated in various
therapeutic indications at the moment. ZYN001, a pro-drug patch that comprises
of THC, is aimed at enabling transdermal delivery via a person’s skin and into
their circulatory system. ZYN002, a CBD gel, is the first of its kind and is
designed to act as a non-psychoactive cannabinoid. The gel is to be delivered
via the skin and absorbed into a patient’s circulatory system. At present, the
gel is in phase 2 of clinical development with patients who suffer with
osteoarthritis of the knee, refractory epilepsy, and Fragile X Syndrome.
Focused on the development and commercialization of innovative therapies that
improve the quality of life of patients, Insys Therapeutics (NASDAQ: INSY) is
developing a pipeline of products intending to address unmet medical needs and
clinical shortcomings of existing commercial products. The Drug Enforcement
Agency recently issued a provisional final ruling that is to result in Insys’
SYNDROS™ being placed in Schedule II of the Controlled Substances Act. The
product has been designed for chemotherapy patients and is aimed at helping to
alleviate vomiting and nausea. It is also aimed at helping AIDS patients
suffering from anorexia-type weight loss.
To date, there are some 53 million outpatients and 45 million inpatient
surgeries that are performed in the United States annually. These surgeries
necessitate drugs for postoperative pain, and more than half of postoperative
patients continue to experience poor pain relief. At present, the therapeutic
classes for postoperative pain management involve analgesics such as
hydromorphone, morphine, and fentanyl, as well as NSAIDs – non-steroidal anti-inflammatories.
Furthermore, the market is awash with injectable opioids that are administered
either via epidural, intravenous, intrathecal, or intramuscular. Usually, the
injections are administered either by patient-controlled analgesia devices or
hospital personnel.
It has been found that the market for effective acute pain management in the
form of combined therapies outside of the hospital setting exceeds more than 200
million prescriptions yearly. While products such as opioid analgesic
combinations make up the bulk of the market, the products have significant
side-effects, including sedations, dizziness, respiratory problems, substance
abuse, euphoria, vomiting, and nausea. Hence, there is a vast market for
combined therapies that are able to manage acute pain without producing the
negative and uncomfortable side effects.
For more information on India Globalization Capital please visit India
Globalization Capital, Inc. (NYSE:
IGC)
About NetworkNewsWire
NetworkNewsWire (NNW) provides news aggregation and syndication, enhanced press
release services and a full array of social communication solutions. As a
multifaceted financial news and distribution company with an extensive team of
contributing journalists and writers, NNW is uniquely positioned to best serve
private and public companies that desire to reach a wide audience of investors,
consumers, journalists and the general public. NNW has an ever-growing
distribution network of more than 5,000 key syndication outlets across the
country. By cutting through the overload of information in today’s market, NNW
brings its clients unparalleled visibility, recognition and brand awareness. NNW
is where news, content and information converge.
NetworkNewsWire (NNW)
New York, NY
www.NetworkNewsWire.com
Please see full disclaimers on the NetworkNewsWire website applicable to all
content provided by NNW, wherever published or re-published: http://NNW.fm/Disclaimer
DISCLAIMER: NetworkNewsWire (NNW) is a source of content listed above.
References to any issuer other than the profiled issuer are intended solely to
identify industry participants and do not constitute an endorsement of any
issuer or comparison to the profiled issuer. FN Media Group, LLC (FNM), is a
third-party publisher and news dissemination service provider, which
disseminates electronic information through multiple online media channels. FNM
is NOT affiliated with NNW or any company mentioned herein. The commentary,
views and opinions expressed in this release by NNW are solely those of NNW and
are not shared by and do not reflect in any manner the views or opinions of FNM.
Readers of this content agree that they cannot and will not seek to hold liable
NNW and FNM for any investment decisions by their readers or subscribers. FNM
and its affiliated companies are a news dissemination and financial marketing
solutions provider and are NOT a registered broker-dealer/analyst/adviser, hold
no investment licenses and may NOT sell, offer to sell or offer to buy any
security. FNM was not compensated by any public company mentioned herein to
disseminate this press release. NNW’s compensation disclosure is incorporated
herein and appears in full at http://NNW.fm/Disclaimer
NNW & FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW & FNM undertakes no obligation to update such
statements.
Media Contact e-mail: FN Media Group, LLC - editor@financialnewsmedia.com
(954)345-0611
News Source: NetworkNewsWire
IGC Files Patent for
Cannabis-based Combination Therapy for Treatment of Eating Disorders
BETHESDA, Md., March 21, 2017 (GLOBE NEWSWIRE) -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
today announces its provisional patent filing (IGC-506) for the treatment of
multiple types of eating disorders by a method and combination of cannabis
extracts and other compounds. IGC-506 is being developed for both human and
veterinary use, should this filing progress to the issuance of a patent.
“The development of cannabis-based combination therapies represents a large,
unique opportunity, and we believe we are the first mover in this emerging
specialty-pharmaceutical sector. Over the past three years, we have built a
strong IP portfolio and now propose to obtain funding to commence pre-clinical
and clinical trials as appropriate. Securing FDA approval for combination
therapies is generally much faster and less expensive than the process for new
drug applications. As a result, we believe that we can bring our cannabis-based
pharmaceutical products to market in both an expeditious and cost-effective
manner,” states Ram Mukunda, chief executive officer of IGC.
As previously reported ( http://nnw.fm/e6A76
), IGC has started a review of international medical facilities to commence
preclinical and clinical trials in support of the Company’s patent portfolio,
which includes IGC-501 for neuropathic pain; IGC-502 for the treatment of
seizures; and IGC-504 for eating disorders, including cachexia.

It should be noted that filing a provisional patent does not guarantee the
issuance of a patent in the future.
Patent Portfolio & Market Application
IGC-501 - indicated for neuropathic pain. The pain market represents a
significant component of the U.S. health care system, costing between $560
billion-$635 billion per year, according to the most recent data from the
Journal of Pain (2012).
IGC-502 - indicated for seizures. Approximately 50 million people worldwide are
affected by epilepsy (Sanders, 2003), a condition attributed to multiple
factors.
IGC-504 and IGC-506- indicated for eating disorders, including cachexia, a
condition that accompanies severe illness such as cancer and results in the
weakness and wasting away of the body. Approximately 1.3 million people in the
United States are experiencing cachexia associated with cancer, multiple
sclerosis, Parkinson’s disease, HIV/AIDS, and other progressive illnesses.
Notably, cancer-induced anorexia/cachexia is responsible for about 20% of all
cancer deaths.
About IGC
India Globalization Capital is engaged in the development of cannabis-based
therapies to treat pain, PTSD, seizures, cachexia, chronic and terminal
neurological and oncological diagnoses, and other life altering conditions. In
support of this mission, IGC has assembled a portfolio of patent filings for its
phytocannabinoid-based treatments. The company is based in Bethesda, Maryland.
For more information visit www.igcinc.us
Follow us on Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements
Some of the statements contained in this press release that are not historical
facts constitute forward-looking statements under the federal securities laws.
Forward-looking statements can be identified by the use of the words "may,"
"will," "should," "could," "expects," "plans," "anticipates," "believes,"
"estimates," "predicts," "intends," "potential," "proposed" or the negative of
those terms. These statements are not a guarantee of future developments and are
subject to risks, uncertainties, and other factors, some of which are beyond
IGC's control and are difficult to predict. Consequently, actual results may
differ materially from information contained in the forward-looking statements
as a result of future changes or developments in IGC's business and acquisition
and diversification strategy, competitive environment, infrastructure demands,
and governmental, regulatory, political, economic, legal and social conditions
in, among other places, China and India. Except as required by federal
securities laws, IGC undertakes no obligation to publicly update any
forward-looking statements, whether as a result of new information or future
events, or otherwise. Other factors and risks that could cause or contribute to
actual results differing materially from such forward-looking statements have
been discussed in greater detail in IGC's Form 10-K for fiscal year ended March
31, 2016, and in subsequent reports filed with the U.S. Securities and Exchange
Commission.
IGC Contact Info:
Claudia Grimaldi
General Manager
301.983.0998 Office
Communications Contact:
NetworkNewsWire (NNW)
New York, New York
www.NetworkNewsWire.com
212.418.1217 Office
Editor@NetworkNewsWire.com
Source: India Globalization Capital, Inc.
Unique Cannabis-Pharma
Companies Trailblazing the Medical Cannabis Industry
New York, NY – March 16th, 2017 –
NetworkNewsWire.com News Coverage:
The cannabis industry has swiftly turned into a multi-billion dollar
opportunity. In the United States, this market’s massive growth potential is
being recognized as
 new
states legalize marijuana for medical or recreational use. Industry experts
forecast the U.S. cannabis market rounding $7 billion in 2016, while the annual
sale of state-legal cannabis products is forecast to exceed $20 billion by 2020.
Within this flourishing market, cannabis-pharma companies like India
Globalization Capital (NYSE:
IGC), Zynerba Pharmaceuticals, Inc. (NASDAQ: ZYNE), OWC Pharmaceutical
Research Corp. (OTC: OWCP), Insys Therapeutics, Inc. (NASDAQ: INSY) and GW
Pharmaceuticals plc (NASDAQ: GWPH) are carving lanes of unprecedented
opportunities in the medical field. new
states legalize marijuana for medical or recreational use. Industry experts
forecast the U.S. cannabis market rounding $7 billion in 2016, while the annual
sale of state-legal cannabis products is forecast to exceed $20 billion by 2020.
Within this flourishing market, cannabis-pharma companies like India
Globalization Capital (NYSE:
IGC), Zynerba Pharmaceuticals, Inc. (NASDAQ: ZYNE), OWC Pharmaceutical
Research Corp. (OTC: OWCP), Insys Therapeutics, Inc. (NASDAQ: INSY) and GW
Pharmaceuticals plc (NASDAQ: GWPH) are carving lanes of unprecedented
opportunities in the medical field.
Bethesda, Maryland-based India Globalization Capital (NYSE:
IGC) is advancing the development of cannabis-based therapies targeting
large market conditions and illnesses. IGC is on a mission to treat chronic and
terminal neurological and oncological diagnoses and other life altering
conditions utilizing an exciting and specialized IP platform that formulates and
tests combination therapies. With this special focus in mind, IGC has amassed a
portfolio of patent filings covering the indications of pain, medical refractory
epilepsy and cachexia (cancer-induced anorexia) using cannabinoids. As we will
further discuss later, IGC has established an approach that significantly
differentiates the company from its cannabis-pharma peers.
Like IGC, Zynerba Pharmaceuticals (NASDAQ: ZYNE) is dedicated to the development
of innovative cannabinoid treatments that target seizures and pain. Unlike IGC
and Cannabics, this clinical-stage, specialty pharmaceutical company is
concentrating on developing transdermal synthetic cannabinoid treatments. On
March 13, 2017, the company announced that it had completed enrolling patients
for Phase 2 of its STAR 1 (Synthetic Transdermal Cannabidiol for the Treatment
of Epilepsy) clinical trial evaluating ZYN002 cannabidiol (CBD) gel in adult
epilepsy patients with refractory focal seizures. Zynerba also revealed that it
had completed enrollment for its Phase 2 STOP (Synthetic Transdermal Cannabidiol
for the Treatment of Knee Pain due to Osteoarthritis) clinical trial evaluating
ZYN002 CBD gel for the treatment of osteoarthritis. Epilepsy) clinical trial evaluating ZYN002 cannabidiol (CBD) gel in adult
epilepsy patients with refractory focal seizures. Zynerba also revealed that it
had completed enrollment for its Phase 2 STOP (Synthetic Transdermal Cannabidiol
for the Treatment of Knee Pain due to Osteoarthritis) clinical trial evaluating
ZYN002 CBD gel for the treatment of osteoarthritis.
OWC Pharmaceutical (OTCQB: OWCP), through its wholly owned One Word Cannabis
Ltd. Israeli subsidiary, is focused on conducting medical research and pursuing
clinical trials to develop cannabis-based pharmaceuticals and treatments for a
variety of conditions. The company is developing two unique delivery systems to
effectively dose and deliver medical cannabis. The first is a proprietary,
cannabinoid-enriched sublingual tablet for the treatment of multiple myeloma,
post-traumatic stress disorder, and fibromyalgia. In in-vitro testing, the
tablets demonstrated 100% malignant cell death in 60% of infected mice cells.
The second product is a proprietary topical compound for the treatment of
psoriasis. In addition, OWC Pharmaceutical operates a Consulting Division
created to help governments and companies navigate complex international
cannabis regulatory frameworks.
Another industry peer, Insys Therapeutics, Inc. (NASDAQ: INSY), is developing
medications to treat addiction to opioids, opioid overdose, epilepsy and other
disease areas with high unmet need. The commercial-stage company uses
proprietary sublingual spray technology to develop pharmaceutical cannabinoids
to address clinical shortcomings of products already on the market. Insys
currently markets its FDA-approved SUBSYS® (fentanyl sublingual spray) for pain
management in cancer patients who are tolerant to opioid therapy. The company
has also received approval for Syndros™ for the treatment of
chemotherapy-induced nausea and vomiting, as well anorexia associated with
weight loss in persons with AIDS. Syndros is an orally administered liquid
formulation of the pharmaceutical cannabinoid dronabinol, a pharmaceutical
version of tetrahydrocannabinol ("THC").
In terms of market share, the giant on the list of cannabis-focused
biopharmaceutical companies is GW Pharmaceuticals (NASDAQ: GWPH), which is
focused on the development of plant-derived cannabinoid therapeutics based on
its proprietary cannabinoid product platform. The company’s primary focus is on
disorders of the central nervous system (CNS). Within this arena, GW’s lead
cannabinoid product candidate is Epidiolex® (cannabidiol), in development for
the treatment of rare childhood-onset epilepsy disorders. The company’s Sativex®
(nabiximols) prescription drug, a therapy approved for the treatment of
spasticity due to multiple sclerosis, is the world’s first plant-derived
cannabinoid prescription drug. Additionally, GW boasts a deep pipeline of
additional cannabinoid product candidates which includes clinical-stage
compounds for glioma, schizophrenia and epilepsy.
It’s an impressive and interesting lineup, but what sets India Globalization
Capital (NYSE:
IGC) apart from the other players is its foray into the larger market
for cannabis-based combination therapies that would treat pain and other
conditions. The company recently filed patents (http://nnw.fm/ggBB6) for
formulations that will reduce the side effects of single drugs by being combined
with a cannabinoid. IGC filed IGC-501 for a cannabis-based formulation to treat
neuropathic and arthritic pain in joints and muscles using a variety of delivery
techniques.
India Globalization Capital also filed combination therapy formulations for the
treatment of epilepsy/seizures (IGC-502) and cachexia (IGC-504). IGC-501,
IGC-502 and IGC-503 are novel combination therapies that, if proven out by
clinical trials, are expected to treat pain, medical refractory epilepsy and
eating disorders respectively, with lower side effects than conventional
therapies.
Such “combination therapies” have the unique opportunity to quickly move through
the FDA’s approval process, since the drugs have already been individually
approved. Consequently, IGC believes it has “first mover” advantage in pursuing
this approach to bring cannabis-based pharmaceutical products to market in a
quick and cost-effective manner.
The cannabis industry is growing in popularity. The wave of enthusiasm
surrounding the recent legalization of cannabis in several new states is
sweeping the country and IGC anticipates an explosion in demand for cannabinoid-based
pharmaceutical therapies, technology, facilities and financial services. With a
solid strategic plan in hand, this NYSE MKT listed company has a modest $9
million market capitalization which is just a fraction of their peer group, and
is aggressively leaning on its connections to medical and procedural expertise
to take advantage of one of many ground-floor opportunities. And, it is
developing innovative therapies, while also acquiring technologies from related
industries that will place it in the right position for capitalizing on its
first-mover advantage.
For more information on India Globalization Capital (IGC) please visit
India Global Capital (IGC) or
http://www.igcinc.us
About NetworkNewsWire
NetworkNewsWire (NNW) provides news aggregation and syndication, enhanced press
release services and a full array of social communication solutions. As a
multifaceted financial news and distribution company with an extensive team of
contributing journalists and writers, NNW is uniquely positioned to best serve
private and public companies that desire to reach a wide audience of investors,
consumers, journalists and the general public. NNW has an ever-growing
distribution network of more than 5,000 key syndication outlets across the
country. By cutting through the overload of information in today’s market, NNW
brings its clients unparalleled visibility, recognition and brand awareness. NNW
is where news, content and information converge.
NetworkNewsWire (NNW)
New York, NY
www.NetworkNewsWire.com
Please see full disclaimers on the NetworkNewsWire website applicable to all
content provided by NNW, wherever published or re-published: http://NNW.fm/Disclaimer
DISCLAIMER: NetworkNewsWire (NNW) is a source of content listed above.
References to any issuer other than the profiled issuer are intended solely to
identify industry participants and do not constitute an endorsement of any
issuer or comparison to the profiled issuer. FN Media Group, LLC (FNM), is a
third-party publisher and news dissemination service provider, which
disseminates electronic information through multiple online media channels. FNM
is NOT affiliated with NNW or any company mentioned herein. The commentary,
views and opinions expressed in this release by NNW are solely those of NNW and
are not shared by and do not reflect in any manner the views or opinions of FNM.
Readers of this content agree that they cannot and will not seek to hold liable
NNW and FNM for any investment decisions by their readers or subscribers. FNM
and its affiliated companies are a news dissemination and financial marketing
solutions provider and are NOT a registered broker-dealer/analyst/adviser, hold
no investment licenses and may NOT sell, offer to sell or offer to buy any
security. FNM was not compensated by any public company mentioned herein to
disseminate this press release. NNW’s compensation disclosure is incorporated
herein and appears in full at
http://NNW.fm/Disclaimer
NNW & FNM HOLDS NO SHARES OF ANY COMPANY NAMED IN THIS RELEASE.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and NNW & FNM undertakes no obligation to update such
statements.
Media Contact e-mail: FN Media Group, LLC - editor@financialnewsmedia.com
(954)345-0611
News Source: NetworkNewsWire
IGC Announces Third Quarter
Financial Results
Transitions Management to Focus on Cannabis-based Therapies
BETHESDA, Md., Feb. 21, 2017 (GLOBE NEWSWIRE) -- India Globalization
Capital, Inc. (NYSE MKT: IGC)
announced financial results for the third quarter ended December 31, 2016 for
the fiscal year that ends March 31, 2017 (the “Q3 2017”).
Revenue for fiscal Q3 2017 was $250,000 compared to $1,085,000 for fiscal Q3
2016. Q3 2017 revenue was primarily generated by renting heavy equipment and
managing the construction of the hotel in Genting, Malaysia. Revenue decreased
because we curtailed trading activity so Management can focus on developing
cannabis-based therapies.
Selling, general and administrative expenses were $323,000 for Q3 2017 as
compared to $438,000 for Q3 2016. The Q3 2017 SG&A primarily reflects expense
reduction initiatives, expenses associated with cannabis-based therapies
development, and public company expenses.

In Q3 2017, the Company reported a GAAP net loss of $111,000 and a GAAP EPS loss
of $0.00, compared to a GAAP net loss of $403,000 and a GAAP EPS loss of $0.02
for Q3 2016. The improved quarterly performance is attributable to Management’s
efforts to focus on developing cannabis-based therapies, and by realization of
cumulative previously deferred foreign exchange gains related to Ironman.
For the period ended December 31, 2016, our cash and cash equivalents along with
restricted cash was approximately $744,000 and our stockholders’ equity was
approximately $7,540,000 compared with approximately $13,948,000 for the period
ended March 31, 2016. In Q3 2017 the reduction in stockholders’ equity was
attributable to giving up control over Ironman assets. This was previously
reported by IGC on Form 8-K on January 6, 2017. On a proforma basis IGC’s
stockholders’ equity as of March 31, 2016, was approximately $6,203,000 versus
actual as of December 31, 2016 of approximately $7,540,000, an increase of
$1,337,000.
“In 2017 our goal is to accelerate the development of our cannabis-based therapy
portfolio to support key indications such as Pain, Seizures, Cachexia, PTSD and
Depression. In tandem, we expect to initiate pre-clinical trials on
IGC-501-Pain, IGC-502-Seizures and IGC-504-Cachexia," stated Ram Mukunda, CEO.
About IGC
In the United States, we develop cannabis-based therapies. IGC has assembled a
portfolio of patent filings that encompasses the indications of Pain, Seizures,
Epilepsy, and Cachexia using phytocannabinoids. We are based in Bethesda,
Maryland.
Our website: www.igcinc.us. Follow us at: Twitter @IGCIR and Facebook.com/IGCIR/
Forward-looking Statements:
Some of the statements contained in this press release that are not historical
facts constitute forward- looking statements under the federal securities laws.
Forward-looking statements can be identified by the use of the words "may,"
"will," "should," "could," "expects," "plans," "anticipates," "believes,"
"estimates," "predicts," "intends," "potential," "proposed," or the negative of
those terms. These statements are not a guarantee of future developments and are
subject to risks, uncertainties and other factors, some of which are beyond
IGC's control and are difficult to predict. Consequently, actual results may
differ materially from information contained in the forward-looking statements
as a result of future changes or developments in our business, our acquisition
and diversification strategy, our competitive environment, and governmental,
regulatory, political, economic, legal and social conditions. Except as required
by federal securities laws, IGC undertakes no obligation to publicly update any
forward- looking statements, whether as a result of new information, future
events, or otherwise. Other factors and risks that could cause or contribute to
actual results differing materially from such forward- looking statements have
been discussed in greater detail in IGC's Form 10- K for fiscal year ended March
31, 2016, and in subsequent reports filed with the U.S. SEC.
SEE FULL FINANCIAL STATEMENTS at
http://finance.yahoo.com/news/igc-announces-third-quarter-financial-231342542.html
Contact:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
India Globalization Capital
Announces the Extension of Warrants’ Expiry Date and Inducement Grant to its CFO
BETHESDA, Md., Feb. 17, 2017 (GLOBE NEWSWIRE) -- India Globalization
Capital, Inc. (NYSE MKT:IGC)
announces the extension of the expiration date for 11,656,668 outstanding
warrants trading on the OTC Markets with ticker symbol IGC.IW and CUSIP number
(45408X118).
The warrants have an exercise price of $5.00 for 1/10 of a share and were
scheduled to expire on March 6, 2017. The expiration date of the warrants has
been extended from 5:00 p.m. New York time on March 6, 2017 to 5:00 p.m. New
York time on Wednesday, March 6, 2019. As was the case prior to the extension,
the warrants are subject to earlier expiration if the Company exercises its
right to call the warrants for redemption. All other terms remain the same.
The Company filed a registration statement with the Securities and Exchange
Commission to register the shares underlining the warrants to permit the
exercise of the warrants. Currently, the Company has such a registration
statement effective. Holders of the warrants will be able to exercise the
warrants for cash since such a registration statement is effective. This
communication shall not constitute an offer to sell or the solicitation of an
offer to buy nor shall there be any sale of the shares underlying the warrants
in any state in which such offer, solicitation or sale would be unlawful prior
to registration or qualification under the securities laws of any such state.
Currently IGC has its Common Stock (IGC) listed with the NYSE MKT and its
Warrants (IGC.IW) trade on the OTC Markets.
As previously disclosed, on November 15, 2016, IGC appointed Mr. John Cherin to
the position of CFO, Treasurer, and Principal Accounting Officer (PAO). As an incentive to join the Company, on
November 14, 2016, IGC’s Board approved an inducement grant to Mr. Cherin of
150,000 restricted shares of IGC’s common stock valued at approximately $51,000,
subject to vesting and approval by the NYSE MKT.
Principal Accounting Officer (PAO). As an incentive to join the Company, on
November 14, 2016, IGC’s Board approved an inducement grant to Mr. Cherin of
150,000 restricted shares of IGC’s common stock valued at approximately $51,000,
subject to vesting and approval by the NYSE MKT.
The grant was made as an inducement that was a material component of the new
CFO's compensation and subsequent acceptance of employment with the Company and
was granted as an employment inducement award pursuant to exemption (a) under
Section 711 of the NYSE Company Guide and approved by the Company's Compensation
Committee.
About IGC
In the United States, we develop phytocannabinoid-based therapies. IGC has
assembled a portfolio of patent filings that encompasses the indications of
Pain, Medical Refractory Epilepsy and Cachexia using cannabinoids. We are based
in Bethesda, Maryland. Our website: www.igcinc.us. Twitter @IGCIR Facebook.com/IGCIR/
Forward-looking Statements
Some of the statements contained in this press release that are not historical
facts constitute forward-looking statements under the federal securities laws.
Forward-looking statements can be identified by the use of the words "may,"
"will," "should," "could," "expects," "plans," "anticipates," "believes,"
"estimates," "predicts," "intends," "potential," "proposed" or the negative of
those terms. These statements are not a guarantee of future developments and are
subject to risks, uncertainties, and other factors, some of which are beyond
IGC's control and are difficult to predict. Consequently, actual results may
differ materially from information contained in the forward-looking statements
as a result of future changes or developments in IGC's business and acquisition
and diversification strategy, competitive environment, infrastructure demands,
and governmental, regulatory, political, economic, legal and social conditions
in, among other places, China and India. Except as required by federal
securities laws, IGC undertakes no obligation to publicly update any
forward-looking statements, whether as a result of new information or future
events, or otherwise. Other factors and risks that could cause or contribute to
actual results differing materially from such forward-looking statements have
been discussed in greater detail in IGC's Form 10-K for fiscal year ended March
31, 2016, and in subsequent reports filed with the U.S. Securities and Exchange
Commission.
Contact Info:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
IGC Launches Phytocannabinoid
Development Committee to Secure Preclinical Initiatives for IGC-501, IGC-502 and
IGC-504
BETHESDA, Md., Feb. 13, 2017 (GLOBE NEWSWIRE) -- India Globalization
Capital, Inc. (NYSE MKT:IGC)
announces today that its Phytocannabinoid Development Committee has started its
review of international medical facilities as per its mandate to commence
preclinical trials in support of IGC’s patent portfolio. The Committee has a
particular focus on Israel, among others, given the Country’s current cannabis
research policies and depth of qualified healthcare organizations.
During the preclinical phase, each compound will undergo toxicity assessment and
development of their pharmacological profile. It is anticipated that these
multi-week initiatives will allow for the collection of important data related
to safety, iterative testing and feasibility.
IGC-501 is indicated for neuropathic pain. The pain market represents a
significant component of the healthcare system and The Journal of Pain in September 2012 reported that the annual estimated
national cost of pain ranges from $560 billion to $635 billion. Additionally,
The American Pain Society recommends that pain be made more visible and be
categorized as the fifth vital sign.
and The Journal of Pain in September 2012 reported that the annual estimated
national cost of pain ranges from $560 billion to $635 billion. Additionally,
The American Pain Society recommends that pain be made more visible and be
categorized as the fifth vital sign.
IGC-502 is indicated for seizures. Approximately 50 million people worldwide are
affected by Epilepsy (Sanders, 2003). Epilepsy is thought to be due to multiple
factors that include Sodium, Potassium, GABA (gamma amino butyric acid) and NMDA
(N-Methyl-d-aspartate). It is believed that to maximally control Epilepsy,
modulation of one or more of these receptors is required and that mono therapy
is adequate in up to 25% of patients.
IGC-504 is indicated for eating disorders. Cachexia is a condition that
accompanies severe illness such as cancer and results in the weakness and
wasting away of the body. In the U.S. it is estimated that a population of
approximately 1.3 million are experiencing Cachexia associated with Cancer,
Multiple Sclerosis, Parkinson’s disease, HIV/AIDS and other progressive
illnesses. Cancer induced Anorexia Cachexia is responsible for about 20% of all
cancer deaths.
About IGC
In the United States, we develop phytocannabinoid-based therapies. IGC has
assembled a portfolio of patent filings that encompasses the indications of
Pain, Medical Refractory Epilepsy and Cachexia using cannabinoids. We are based
in Bethesda, Maryland.
Our website: www.igcinc.us. Twitter @IGCIR Facebook.com/IGCIR/
Forward-looking Statements
Some of the statements contained in this press release that are not historical
facts constitute forward-looking statements under the federal securities laws.
Forward-looking statements can be identified by the use of the words "may,"
"will," "should," "could," "expects," "plans," "anticipates," "believes,"
"estimates," "predicts," "intends," "potential," "proposed" or the negative of
those terms. These statements are not a guarantee of future developments and are
subject to risks, uncertainties, and other factors, some of which are beyond
IGC's control and are difficult to predict. Consequently, actual results may
differ materially from information contained in the forward-looking statements
as a result of future changes or developments in IGC's business and acquisition
and diversification strategy, competitive environment, infrastructure demands,
and governmental, regulatory, political, economic, legal and social conditions
in, among other places, China and India. Except as required by federal
securities laws, IGC undertakes no obligation to publicly update any
forward-looking statements, whether as a result of new information or future
events, or otherwise. Other factors and risks that could cause or contribute to
actual results differing materially from such forward-looking statements have
been discussed in greater detail in IGC's Form 10-K for fiscal year ended March
31, 2016, and in subsequent reports filed with the U.S. Securities and Exchange
Commission.
Contact Info:
Claudia Grimaldi
301-983-0998
Source: India Globalization Capital
-------------------------------------------------------------------
About India Globalization Capital, Inc.:
Our mission is to treat pain, PTSD, seizures, cachexia, chronic and terminal
neurological and oncological diagnoses, and other life altering conditions with
phytocannabinoid-based treatments.
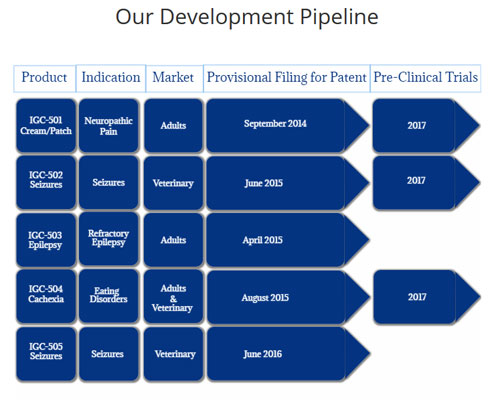
We believe that the legalization of the cannabis industry will create an
explosion in demand for cannabinoid-based pharmaceutical therapies, technology,
facilities, and financial services. While early, we expect to have a first mover
advantage by developing novel therapies, applying for patents, and acquiring
technologies from related industries to crossover to cannabis once federal laws
permit.
Our Strategy
IGC is developing a product portfolio of phytocannabinoid-based therapies
through leading edge research for the treatment of a wide range of therapeutic
indications. These include neuropathic and cancer pain, epilepsy, end of life
supportive care, adjunctive supportive therapies of chronic neurological and
oncological diagnosis, which are life altering or life threatening.
Our Model
We deploy an outsourced model that leverages our international presence and
allows us to control and seek out cost effective solutions. Our team includes an
international network of doctors, veterinarians, and researchers with extensive
expertise in various indications, medicines, controlled substances, plant-based
products, extraction technologies, and drug delivery technologies. In addition
we have assembled experts in patent law, clinical trials, and regulatory
procedures.
Our Alliances
We recognize that there is considerable knowledge at the grass roots level of
the cannabis industry. We are seeking information exchanges with owners of
medical cannabis dispensaries and practitioners with experience in cannabis
based medicines and nutraceuticals.
Cannabis History
An understanding of the subspecies of the cannabis plant is imperative for a
well-rounded comprehension of the history of cannabis and the role it plays in
today’s modern era. Cannabis sativa, often described as “marijuana,” has
psychoactive properties. On the contrary, Cannabis sativa L., commonly referred
to as “hemp,” is a non-psychoactive form of cannabis. Further psychoactive
species of cannabis are found in Cannabis indica and Cannabis ruderalis.
Notably, however, Cannabis ruderalis is rarely used recreationally due to low
Tetrahydrocannabinol (THC) content; these strains are high in the cannabinoid
Cannabidiol (CBD), and hence are often used by medical users. Cannabis has often
been described as a plant that follows humans. It is perhaps for this reason
that the history of cannabis is extensive and dates back thousands of years.
Current State of Affairs
In a recent CNN interview with Dr. Sanjay Gupta as part of "Weed 3", President
Barack Obama was asked “[t]here is a bill on the floor of the Senate now,
proposing that marijuana get rescheduled from Schedule 1 (I) to Schedule 2 (II)
– saying it has no medicinal benefit to possible medicinal benefit. Do you
support that?” President Obama responded: You know, I think I'd have to take a
look at the details, but I’m on record as saying that not only do I think
carefully prescribed medical use of marijuana may in fact be appropriate and we
should follow the science as opposed to ideology on this issue, but I’m also on
record as saying that the more we treat some of these issues related to drug
abuse from a public-health model and not just from an incarceration model, the
better off we’re going to be. The significance of this statement by President
Obama should not be underestimated; it is indicative of the constantly changing
attitudes towards medical and recreational cannabis regulation across the
nation.
Cannabis Politics and Overview of Bills in Congress
Attitudes towards cannabis have transformed substantially across the U.S.
Support for cannabis legalization is rapidly outpacing opposition; 53 percent of
Americans believe cannabis should be made legal, compared with 44 percent
supporting illegality of cannabis. However, not all segments of society support
legalization. Approximately 39 percent of Republicans support legalization,
compared to 63 percent of Democrats. Opposition to legalization is much higher
among those aged 65 and older, and among Hispanics than non-Hispanic whites or
blacks.49 Majorities across nearly all partisan and demographic groups believe
possession of small amounts of cannabis should not result in jail time.
Market Size
The Colorado cannabis market is estimated to consume between 104.2 and 157.9
metric tons (MT) per year, accounting for Colorado residents and visitors.75
These numbers have been suggested using demand- and supply-oriented models
limiting scope only to those aged 21 and over, although use among under-21s is
widespread. Notably, demand-oriented formulae have been favored across the board
for various reasons, including the lack of availability of data when using a
supply-side approach. For this reason, discussions of this segment will adhere
to a demand-based approach. In a demand-oriented approach, the number of users
and the quantity of cannabis consumed by various users are estimated over a
specified period of time, typically one month. Users are then subdivided based
on frequency of use per month. Furthermore, the quantity demanded is tallied
based upon dosage estimates, which can vary widely.
Obstacles Faced by the Industry
Banking is likely the single most precarious issue for the legal cannabis
industry. Since cannabis is still listed under CSA as
 a
Schedule 1 (I) substance, it remains a federal-level crime to use, possess, and
distribute it. This triggers anti-money laundering laws for banks. The Bank
Secrecy Act (BSA), enforced by the Financial Crimes Enforcement Network (FinCEN),
requires banks to monitor customer accounts for suspicious activity associated
with crime and terrorism. BSA requires banks to investigate their customers
thoroughly and neither negligently nor knowingly conduct business with those
acting illegally. FinCEN requires that financial institutions file Suspicious a
Schedule 1 (I) substance, it remains a federal-level crime to use, possess, and
distribute it. This triggers anti-money laundering laws for banks. The Bank
Secrecy Act (BSA), enforced by the Financial Crimes Enforcement Network (FinCEN),
requires banks to monitor customer accounts for suspicious activity associated
with crime and terrorism. BSA requires banks to investigate their customers
thoroughly and neither negligently nor knowingly conduct business with those
acting illegally. FinCEN requires that financial institutions file Suspicious
Strains
Cannabis is grown in many strains. Each strain has a different level or ratio of
cannabinoids that are a class of compounds that act on the cannabinoid
receptors. Phytocannabinoids are naturally occurring cannabinoids found in the
cannabis plant. There are over 60 different cannabinoids that have been isolated
from the cannabis plant that exhibit varied effects. The role of most of these
cannabinoids is yet to be fully understood.
Delta 9 THC
The most commonly known, studied, and characterized cannabinoids are
Tetrahydrocannabinol or THC, and Cannabidiol or CBD. THC (or its main isomer
Delta-9 THC) was first isolated by Israeli scientists in 1964. It is responsible
for the psychotropic effect (“high”) reported by cannabis users. Its synthetic
form is prescribed in the U.S. and Canada under the brand name Marinol. The
active ingredient Dronabinol (synthetic Delta-9 THC) is a light- yellow resinous
oil that is formulated in sesame oil and administered in capsules. It is used to
treat nausea and vomiting caused by cancer chemotherapy and loss of appetite and
weight loss in HIV patients.
Cannabinoid Medicinal Properties
Source: http://loproconsulting.com/
CBD & Charlotte's Web
Based on the strain of the cannabis plant, as much as 40% of the plant’s extract
is cannabidiol or CBD. This phytocannabinoid appears to have a wider scope in
medical applications than THC, which has attracted much more media attention.
Unlike THC, CBD has no psychotropic effects. Though it has not been
scientifically tested, CBD is believed (and has been anecdotally shown) to have
various anti-psychotic, neuroprotective, anti-convulsive, anxiety-reductive, and
anti-depressive properties. (Charlotte’s web for example is one of the high
profile strains of cannabis that has a higher ratio of CBD to THC). Like the
marijuana plant, the hemp plant is also a good source of CBD, and the hemp
extract does not contain THC.
Ligands
THC and CBD and other cannabinoids act as ligands. Imagine a receptor as a
docking station and the class of ligands, small molecules with special
properties, as little computers that dock with the docking station. The effect
of this docking is to activate the receptor in a prescribed way, which then sets
off the downstream biological behavior. Ligands can activate receptors
positively (full agonists) or negatively (inverse agonists). Phytocannabinoids
(like endocannabinoids) are ligands that are considered to be partial agonists
as they play a milder modulatory role.
Research
Much of the research focuses on discovering ligands that can interact with
receptors associated with particular disorders. Phytocannabinoids are more
interesting because they do more than interact with receptors. They interact
with a range of cellular pathways that are implicated in cancer and other
diseases. Thus the elevated interest in using phytocannabinoid based drugs.
Our Strategy
IGC’s strategy is to work with our network of specialists to discover novel
applications of phytocannabinoids for pharmaceutical and nutraceutical therapies
and products. Our focus is less on discovering ligands or ligand-receptor
interactions and entirely on therapies that activate multiple receptors,
pathways, and processes.
We are dedicated to keeping our present and potential shareholders informed with
the latest news and relevant information.
Investor FAQs
Q: What is the stock symbol for the Company and where is it listed?
The stock symbol is IGC and we are listed on the NYSE MKT.
Q: Tell me about the warrants.
There are 11,652,648 public IGC warrants that allow holders to purchase
1,165,264 shares of common stock for an aggregate price of $50 per share. The
warrants are listed as IGC.WT. The warrants expire on March 6, 2017.
Q: Where is IGC incorporated?
We are a Maryland based company located in Bethesda Maryland, USA.
Q: Who does IGC use for clearing its stock?
We use Continental Stock Transfer & Trust Company.
Q: How do I obtain the latest annual report?
To receive a copy of IGC’s annual report, please go to sec.gov.
Q: Does IGC’s financial year end on December 31?
No, our financial year ends on March 31.
Corporate Governance
Audit Committee:
Mr. Richard Prins (Chairman) and Mr. Sudhakar Shenoy
Compensation Committee:
Mr. Sudhakar Shenoy (Chairman) and Mr. Richard Prins
Nominating Committee:
Full board
IGC has adopted its Code of Ethics and Audit Committee Charter to help ensure
that it retains its integrity and merits public trust and confidence.
SOURCE: http://igcinc.us/
Disclaimer
FN Media Group LLC (FNMG) owns and operates
FinancialNewsMedia.com (FNM)
which is a third party publisher that disseminates electronic information
through multiple online media channels. FNMG's intended purposes are to deliver
market updates and news alerts issued from private and publicly trading
companies as well as providing coverage and increased awareness for companies
that issue press to the public via online newswires. FNMG and its affiliated
companies are a news dissemination and financial marketing solutions provider
and are NOT a registered broker/dealer/analyst/adviser, holds no investment
licenses and may NOT sell, offer to sell or offer to buy any security. FNMG's
market updates, news alerts and corporate profiles are NOT a solicitation or
recommendation to buy, sell or hold securities. The material in this release is
intended to be strictly informational and is NEVER to be construed or
interpreted as research material. All readers are strongly urged to perform
research and due diligence on their own and consult a licensed financial
professional before considering any level of investing in stocks. The companies
that are discussed in this release may or may not have approved the statements
made in this release. Information in this release is derived from a variety of
sources that may or may not include the referenced company's publicly
disseminated information. The accuracy or completeness of the information is not
warranted and is only as reliable as the sources from which it was obtained.
While this information is believed to be reliable, such reliability cannot be
guaranteed. FNMG disclaims any and all liability as to the completeness or
accuracy of the information contained and any omissions of material fact in this
release. This release may contain technical inaccuracies or typographical
errors. It is strongly recommended that any purchase or sale decision be
discussed with a financial adviser, or a broker-dealer, or a member of any
financial regulatory bodies. Investment in the securities of the companies
discussed in this release is highly speculative and carries a high degree of
risk. FNMG is not liable for any investment decisions by its readers or
subscribers. Investors are cautioned that they may lose all or a portion of
their investment when investing in stocks. This release is not without bias, and
is considered a conflict of interest if compensation has been received by FNMG
for its dissemination. To comply with Section 17(b) of the Securities Act of
1933, FNMG shall always disclose any compensation it has received, or expects to
receive in the future, for the dissemination of the information found herein on
behalf of one or more of the companies mentioned in this release. For current
services performed FNMG has been compensated one thousand nine hundred dollars for
India Globalization Capital, Inc.. coverage by
a non affiliated third party. FNMG HOLDS NO SHARES OF India Globalization Capital, Inc.
This release contains "forward-looking statements" within the meaning of Section
27A of the Securities Act of 1933, as amended, and Section 21E the Securities
Exchange Act of 1934, as amended and such forward-looking statements are made
pursuant to the safe harbor provisions of the Private Securities Litigation
Reform Act of 1995. "Forward-looking statements" describe future expectations,
plans, results, or strategies and are generally preceded by words such as "may",
"future", "plan" or "planned", "will" or "should", "expected," "anticipates",
"draft", "eventually" or "projected". You are cautioned that such statements are
subject to a multitude of risks and uncertainties that could cause future
circumstances, events, or results to differ materially from those projected in
the forward-looking statements, including the risks that actual results may
differ materially from those projected in the forward-looking statements as a
result of various factors, and other risks identified in a company's annual
report on Form 10-K or 10-KSB and other filings made by such company with the
Securities and Exchange Commission. You should consider these factors in
evaluating the forward-looking statements included herein, and not place undue
reliance on such statements. The forward-looking statements in this release are
made as of the date hereof and FNMG undertakes no obligation to update such
statements.
|





